Abstract
We examined the characteristics of food allergy prevalence and suggested the basis of dietary guidelines for patients with food allergies and atopic dermatitis. A total of 2,417 patients were enrolled in this study. Each subject underwent a skin prick test as well as serum immunoglobulin E (IgE) measurement. A double-blind, placebo-controlled food challenge was conducted using milk, eggs, wheat, and soybeans, and an oral food challenge was performed using beef, pork, and chicken. Food allergy prevalence was found among 50.7% in patients with atopic dermatitis. Among patients with food allergies (n = 1,225), the prevalence of non-IgE-mediated food allergies, IgE-mediated food allergies, and mixed allergies was discovered in 94.9%, 2.2%, and 2.9% of the patients, respectively. Food allergy prevalence, according to food item, was as follows: eggs = 21.6%, milk = 20.9%, wheat = 11.8%, soybeans = 11.7%, chicken = 11.7%, pork = 8.9% and beef = 9.2%. The total number of reactions to different food items in each patient was also variable at 45.1%, 30.6%, 15.3%, 5.8%, 2.2%, and 1.0% for 1 to 6 reactions, respectively. The most commonly seen combination in patients with two food allergies was eggs and milk. The clinical severity of the reactions observed in the challenge test, in the order of most to least severe, were wheat, beef, soybeans, milk, pork, eggs, and chicken. The minimum and maximum onset times of food allergy reactions were 0.2-24 hrs for wheat, 0.5-48 hrs for beef, 1.0-24 hrs for soybeans, 0.7-24 hrs for milk, 3.0-24 hrs for pork, 0.01-72 hrs for eggs, and 3.0-72 hrs for chicken. In our study, we examined the characteristics of seven popular foods. It will be necessary, however, to study a broader range of foods for the establishment of a dietary guideline. Our results suggest that it may be helpful to identify food allergies in order to improve symptoms in patients with atopic dermatitis.
Atopic dermatitis (AD) is a chronic inflammatory disease of the skin most commonly diagnosed in children; however, it can occur at any age. The prevalence of AD has been increasing in Korea, at a rate of 19.7% in 1995 to 27.5% in 2000; in 2003 and 2006, the rate soared to 33.1% among elementary school children and 34.9% among preschoolers, respectively [1-3]. These rapid increases are most likely due to environmental changes, although there is a clear genetic component in AD. It has been reported that a reduced barrier function as well as an altered immune function are fundamental causes of the development of AD [4].
Food allergies (FA) arise from a specific reproducible immune response that occurs with exposure to a given food [4]. The clinical spectrum of FA ranges from mild skin irritation to severe, life-threatening anaphylaxis. Currently, avoidance of the causative agent remains as the mainstay of FA management [4,5]. Food allergic or immunologic reactions are classified into three groups, including IgE-mediated food allergies (IFAs), non-IgE-mediated food allergies (NFAs), and mixed food allergies (NFA + IFA) [6]. NFAs were slower than IFAs in onset time and primarily resulted in gastrointestinal reactions. IFAs demonstrated rapid onset, with most cases resulting in anaphylaxis or urticaria. Table 1 shows the classification of NFA and IFA patients based on skin manifestations and time of onset.
The majority of FAs are mediated by IgE yet, sensitization to a specific food, as confirmed by the skin prick test (SPT) or serum-specific IgE, does not always imply clinical reactivity. Accurate diagnosis of FAs should be based on an open food challenge (OFC) [7]. Because there are no specific laboratory tests for NFAs, diagnoses are made using elimination diets and oral challenges [8,9], as well food challenge tests for confirmation of the FA [10,11]. Several food challenge methods have been proposed, including double-blind, placebo-controlled food challenges (DBPCFC) and OFCs [12]. In an OFC, the patient is not blinded and thus recognizes the test food. The test results are then interpreted according to the objective symptoms. If the patient displays anxiety about the challenge and complains of abdominal pain or pruritus, the food challenge test is considered invalid [13]. DBPCFC is considered to be a more accurate method for diagnosing FAs, as both the patient and the observer are blinded to the test food. This is the most rigorous method for diagnosing adverse food reactions [14]; however, it is difficult to perform DBPCFCs in a clinical setting.
In Japan, 70% of all patients who required treatment for acute allergic reactions had food allergies to eggs, milk, or wheat [15]. Ellman et al. [16] reported in the U.S., allergies to eggs, milk, wheat, soy, peanuts, tree nuts, and seafood account for up to 90% of food allergies in children with AD. In Korea, the prevalence of food allergies by OFC testing among patients with AD was as follows: milk; 67.3%, chicken; 64.2%, pork; 62.8%, eggs; 61.0%, beef; 55.4%, wheat; 52.0% and soybeans; 45.2%. Little research has been conducted on these 7 specific foods that have been shown to be the primary food allergens in Korea [17]. The aim of the present study was to investigate the characteristics of FAs in patients with AD. We determined the prevalence of types of food allergies, the prevalence of combinations of food allergies, the SCORing Atopic Dermatitis (SCORAD) index, and the onset time of allergic reaction in patients with AD.
A total of 2,417 patients (M:F = 1,222:1,195) who visited either the Department of Allergy and Clinical Immunology at the Seoul Allergy Clinic, Seoul, Korea or the Chungnam National University Hospital Pediatric Allergic Clinic, Daejeon, Korea between June 2000 and August 2010 were enrolled in this study (Table 2). The age groups were classified with respect to the physiological characteristics and developmental stages of infants, toddlers, children, adolescents, adults, and the elderly, as referenced by the age classification of the Korean Nutrition Society [18]. All subjects suffered from repeated eczematous reactions or exacerbations of AD. The subjects received blood tests and skin prick tests (SPTs), as described below, and fulfilled the criteria of Hanifin and Rajka [19]. Informed consent was obtained from adult patients or the parents of children participating in the study. This study was approved by the Institutional Review Board of Chungnam University, Daejeon, Korea.
All subjects underwent blood tests and SPTs. Blood tests included a complete blood cell count (CBC) with differential in order to determine the eosinophilic fraction, total serum IgE levels, and food specific serum IgE levels (which were measured using UniCAP; Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden). Serum food-specific IgE levels less than 0.35 kU/L were classified as undetectable.
SPTs were simultaneously conducted on patients' left forearm using crude and commercial allergy extracts (Bencard, Brentford, England). Histamine hydrochloride (1 mg/ml, Bencard) was used as a positive control. SPTs using vehicles (physiologic saline, distilled water, and glycerol) were the negative controls. Reactions were read 15 min after the skin prick test and were classified as either negative (0; no reaction, +; reaction greater than the control reaction but smaller than half the size of histamine) or positive (++; half, +++; same, and ++++; twice the size of histamine). The minimum size for a positive reaction was 3 mm (+++).
Food allergens (1:10 or 1:20 glycerinated food extract) that elicited a wheal at least 3 mm larger than the negative control (not including erythema) were considered to be positive, while anything less than 3 mm was considered to be negative.
Diagnoses of allergies to milk, eggs, wheat, soybeans, beef, pork, and chicken were made for all subjects via food challenge tests. Among the seven foods, milk, eggs, wheat, and soybeans were tested using DBPCFCs, whereas beef, pork and chicken were tested via OFCs. Food allergies were classified as IFA, NFA, and mixed types, based on the objective symptoms, as described in Table 1.
A trial of an elimination diet preceded the food challenge test for all 7 foods. Patients were asked to eliminate the suspected allergen according to their past history of allergic responses, skin prick tests, and specific IgE (maximum elimination phase). A replacement diet was designed in order to provide substitutes for the eliminated foods in order to maintain a balanced nutrition [17]. To confirm the complete elimination of all foods identified as allergens, all patients were required to keep dietary diaries. The analysis of dietary diaries was performed by dietitians as well as physicians. In addition, the elimination diet was implemented to the mother's diet for breast fed infants.
OFCs were performed according to the following indications (challenge phase): a) obvious clinical improvement was obtained and the clinical status was stable for at least 2 weeks, and b) the allergens tested were completely eliminated from the diet. This was confirmed via the analysis of the dietary diary by a dietician. To exclude possible environmental factors, patients maintained their daily living patterns as regularly as possible during the testing period.
Patients consumed one portion of the test food each morning [18]. Three days after the first challenge, the clinical results and the severity score were evaluated. If patients showed increased severity scores or obvious worsening of clinical signs or symptoms, the tests were discontinued. Otherwise, the food challenge tests continued with ingestion of increased quantities of the test food for another 4 days. If patients showed worsening symptoms, the subsequent challenges were delayed until patients recovered to the pre-challenge state. If patients consumed the food that was to be eliminated during the study, the challenge tests were ceased and the patients were observed for 1 week.
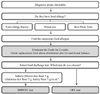
The recipes for DBPCFCs using eggs, milk, and soybeans were developed by dieticians at the Seoul Allergy Clinic. The vehicle used was 15 g of mixed cereal flour, consisting of brown rice flour (5 g), glutinous rice flour (5 g), and barley flour (5 g). For the DBPCFC, a 21 g mixture, consisting of 15 g of the above mixed cereal flour and 6 g of freeze-dried eggs, soybeans, or skim milk powder, was used. Foods that contained eggs, milk, and soybeans were eliminated from daily diets for at least two weeks prior to testing. Before starting the DBPCFC, it was confirmed that the patients tolerated mixed cereal flour by challenging them with the placebo for 7 days. If no symptoms were reported, patients proceeded with the DBPCFC. Two challenges, separated by a period of 7 days, were performed including one with placebo only and one with the suspected food antigen within the vehicle. Fig. 1 summarizes the diagnostic flow of the food challenge tests in patients with atopic dermatitis.
The clinical severity of NFA was assessed using the SCORAD index, a system used worldwide to assess the severity of atopic eczema [20]. A diagnosis of FA was made if the clinical severity score increased by > 20% in subjects demonstrating a baseline score > 50 points, or if the score increased by > 10 points for those with a baseline score ≤ 50 points. New skin lesions and itching were regarded as positive reactions, which were required for the diagnosis of FA by the food challenge test. The clinical severity of IFA was estimated using a slightly modified Clark's scoring system [21]. Positive reactions were assigned 5 points, onset times < 15 min were assigned 10 points, and onset times > 15 min were assigned five points. Clinical severity scores for IFA ranged from 10 to 30 points.
The prevalence of food allergies was 50.7% (1,225/2,417) in patients with AD (Table 2). Patients aged 0-11 months and over the age of 30 showed a FA prevalence of less than 50%. However, patients aged 1-29 years showed a FA prevalence from 53.8%-61.1%, which is higher than other age groups. The type of FA was classified as NFA, IFA, or mixed. The prevalence of NFA was 94.9% (1,162/1,225), IFA was 2.2% (27/1,225), and mixed was 2.9% (36/1,225). Subjects aged 65 and older were presented with only NFA, However, all other age groups showed a combination of NFA, IFA, and mixed (Table 2).
The prevalence of FAs to the 7 food items tested in patients with AD were eggs; 21.6% (523/2,417), milk; 20.9% (506/2,417), wheat; 11.8% (285/2,417), soybeans; 11.7% (282/2,417), chicken; 11.7% (283/2,417), pork; 8.9% (216/2,417), and beef; 9.2% (222/2,417) (Table 3). The most prominent type of FA was NFA, however, milk and eggs more commonly caused IFA when compared to other foods. Beef did not cause IFA.
Among patients with FAs, the prevalence of allergies to the various numbers of food items is shown in Table 4. The prevalence of reactions to 1 food was 45.1% (553/1,225); to 2 foods, 30.6% (375/1,225); to 3 foods, 15.3% (187/1,225); to 4, foods was 5.8% (71/1,225) to 5 foods, 2.2% (27/1,225); and to 6 foods, 1.0% (12/1,225).
We examined the characteristics of 2 FA combinations (Table 5), and found that 28.5% (149/523) of FA patients allergic to eggs were also allergic to milk. Likewise, 29.4% (149/506) of FA patients allergic to milk also were allergic to eggs. Patients with an allergy to one of the other 5 foods tested had a concomitant allergy for eggs or milk more frequently than other foods. For example, 43.5% (124/285) of FA patients with wheat allergy, 42.0% (119/283) with chicken allergy, 41.7% (90/216) with pork allergy, 39.2% (87/222) with beef allergy, and 38.3% (108/282) with soybean allergy also had egg allergy.
Table 6 presents the prevalence of allergies to all 7 food items according to age. In all ages, the prevalence rates of allergy to eggs and milk were higher than that of any other foods. In particular, FA prevalence of milk in patients aged 0-5 months and 6-11 months showed a rate between 14.8-24.7%, which is the lowest rate among all age groups, whereas patients over the age of 1 year showed a FA prevalence of 37.5-55.1%. FA prevalence of soybean in patients aged 6-11 months showed 34.8% which is the highest rate among all age groups.
Table 7 shows the score differences for clinical severity during the food challenge test. The score differences were obtained by subtracting the scores of the pre-challenge test from those of the post-challenge test. The results ranked from greatest to least: wheat, beef, soybeans, milk, pork, eggs, and chicken.
With regards to the mean onset time of the FA reaction, the shortest time to reaction was seen in wheat (6.7 ± 8.2 h), and the longest was seen in chicken (45.3 ± 22.2 h). The minimum and maximum onset time of FA reactions were different between food items: wheat (min = 0.20 h; max = 24 h), beef (0.50 h; 48 h), soybeans (1.0 h; 24 h), milk (0.70 h; 24 h), pork (3.0 h; 24 h), eggs (0.01 h; 72 h),and chicken (3.0 h; 72 h) (Table 7).
Over the last several decades, studies involving controlled food challenges have provided a reliable scientific understanding of the role of foods in hypersensitivity reactions. Most of these investigations have focused on pediatric populations, and as a result, less evidence for adverse reactions to foods in adults has been reported. In our study, the prevalence of FAs was 50.7% (1,225/2,417) in patients with AD. We also examined changes in the prevalence of FAs by age. Less than 40% of patients aged 0-11months and over the age of 30 demonstrated FAs. This is consistent with previous reports that the prevalence of FA in infants with atopic dermatitis ranges from 30-40% [15,16,22]. Patients aged 1-29 years, however, showed a FA prevalence of more than 50%. These results are consistent with another study [23] in which the prevalence of FAs increased with age (1-18 years) in Australia, China, Taiwan, Japan, and Malaysia. Overall, food allergies tended to begin at an early age and were more frequent in children than in the adult population. Our data suggest that the prevalence of FAs gradually increases between the age of 1-2 and declines after the age of 19-29.
The types of FAs were classified as NFA, IFA, or mixed. Among patients with AD, the major type of FA was NFA (94.9%, 1162/1,225), whereas IFA (2.2%, 27/1,225) and mixed (2.9%, 36/1,225) were significantly less common. Subjects older than 65 years of age were only presented with NFA; however, all other age groups showed a combination of NFA, IFA, and mixed. Eigenmann et al. [24] reported that about 35% of children with AD have IFA, as determined by DBPCFC. Additionally, some studies [25,26] have shown the FA prevalence in infants with AD to be 30%. The most common allergy, among the seven foods (milk, eggs, wheat, soybean, beef, pork, and chicken), tested in our study was eggs (21.6%), followed closely by milk (20.9%). The FA that was least frequently observed was pork (8.9%). A previous study demonstrated positive FA rates of 36.7% to egg whites, 34.8% to wheat, 31.7% to milk, and 30.4% to soybeans [27]. As determined by DBPCFC, the prevalence of specific FAs varies according to different reports. For example, the prevalence of milk allergies in patients with AD was reported to be 33.3% or 50% in two separate studies [28,29]. Likewise, the prevalence of soybean allergies was 11.7% in our study; however, this varied widely from 33.3% [28], to 28% [29], and to 3.1% [30] in other studies. Milk and eggs have been reported to be the most frequent causes for food allergies in AD, with the highest rates belonging to egg allergies [25,31-35]. NFA was overall the most common type of FA observed in our study; however, eggs and milk showed higher IFA responses compared to other foods. Interestingly, only beef did not show an IFA response, and the prevalence of FA to meat (beef, pork, and chicken) was lower than that of other foods (milk, eggs, wheat, and soybeans). Eggs and milk are often components of creamy salad dressings, pastas, whipped cream, icing, batters, and many other food preparations. As a result, many patients with these food allergies are often unable to eat baked goods. These are important allergies to be aware of, as reactions may occur immediately and range from mild to life threatening, in cases of anaphylaxis. In this study, the prevalence of milk allergy sharply increased from 1-2 years. The increase is attributed to whole milk consumption, which begins after breastfeeding or the consumption of modified milk formula. Cow's milk is usually the first food given to an infant, and cow's milk hypersensitivity is often the first symptom of an atopic condition. Cow's milk allergy often disappears before the age of 1 year. Associated reactions to other foods develop in approximately 45% of patients [36].
Among FA patients, the prevalence of 1 FA was 45.1% and 2 FAs was 30.6%. Additionally, the prevalence of more than 3 FAs was 15.3%. The food combination which showed the highest prevalence of two FAs was egg and milk in both NFA and IFA type reactions. Thus, patients with FAs have a high probability of having egg and milk allergies.
The food which showed the most severe reaction and the earliest onset time was wheat. Chicken, on the other hand, had the least severe reaction and the latest onset time. In many previous studies, most of the reactions occurring during the OFC were immediate, while delayed reactions were more controversial [37,38]. In other studies, skin manifestations continued to appear up to 7 days after exposure [38,39].
Our results suggest that the management of FAs is vital for improving symptoms of AD. Over the course of the food challenge, nutritional management was required, and dietary guidelines were collectively developed by the patient, dietitian, and physician. Reactions to food items also had variable onset times and levels of severity. As our study examined only 7 common foods, further research will be necessary for the development of a more comprehensive dietary guideline.
References
1. Oh JW, Kim KE, Pyun BY, Lee HR, Choung JT, Hong SJ, Park KS, Lee SY, Song SW, Kim CH, Ahn KM, Nam SY, Shon MH, Kim WK, Lee MH, Kwon BC, Choi SY, Lee SY, Lee HB, Lee SI, Lee JS. Nationwide study for epidemiological change of atopic dermatitis in school aged children between 1995 and 2000 and kindergarten aged children in 2003 in Korea. Pediatr Allergy Respir Dis. 2003. 13:227–237.
2. Nam SY, Yoon HS, Kim WK. Prevalence of allergic disease in kindergarten age children in Korea. Pediatr Allergy Respir Dis. 2005. 15:439–445.
3. Son KY, Park KS, Hwang HH, Yun BS, Lee SJ, Kim MA, Park JY, Kim KE, Jang KC. Prevalence of allergic diseases among primary school children in Ilsan, Gyeonggi and changes of symptoms after environmental control in 2005. Pediatr Allergy Respir Dis. 2007. 17:384–393.
4. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011. 31:61–75.

5. Ebisawa M. Management of food allergy in Japan "food allergy management guideline 2008 (revision from 2005)" and "guidelines for the treatment of allergic diseases in schools". Allergol Int. 2009. 58:475–483.

7. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, Sicherer S, Golden DB, Khan DA, Nicklas RA, Portnoy JM, Blessing-Moore J, Cox L, Lang DM, Oppenheimer J, Randolph CC, Schuller DE, Tilles SA, Wallace DV, Levetin E, Weber R. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008. 100:S1–S148.

8. Bock SA, Lee WY, Remigio L, Holst A, May CD. Appraisal of skin tests with food extracts for diagnosis of food hypersensitivity. Clin Allergy. 1978. 8:559–564.

9. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.
10. Majamaa H, Miettinen A, Laine S, Isolauri E. Intestinal inflammation in children with atopic eczema: faecal eosinophil cationic protein and tumour necrosis factor-alpha as non-invasive indicators of food allergy. Clin Exp Allergy. 1996. 26:181–187.

11. Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984. 74:26–33.

12. Rancé F, Juchet A, Brémont F, Dutau G. Correlations between skin prick tests using commercial extracts and fresh foods, specific IgE, and food challenges. Allergy. 1997. 52:1031–1035.

13. Ito K, Urisu A. Diagnosis of food allergy based on oral food challenge test. Allergol Int. 2009. 58:467–474.

14. Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, Knulst AC, Moneret-Vautrin DA, Nekam K, Niggemann B, Osterballe M, Ortolani C, Ring J, Schnopp C, Werfel T. European Academy of Allergology and Clinical Immunology. Standardization of food challenges in patients with immediate reactions to foods--position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004. 59:690–697.

15. Imai T. National surveillance of immediate type food allergy in Japan. Work Report of a Study for the Development and Prevention of Food Allergy. 2007. Tokyo: Ministry of Health, Labour and Welfare;5–9.
16. Ellman LK, Chatchatee P, Sicherer SH, Sampson HA. Food hypersensitivity in two groups of children and young adults with atopic dermatitis evaluated a decade apart. Pediatr Allergy Immunol. 2002. 13:295–298.

17. Lee SS, Lee KY, Noh G. The necessity of diet therapy for successful interferon-gamma therapy in atopic dermatitis. Yonsei Med J. 2001. 42:161–171.

18. The Korean Nutrition Society. Dietary Reference Intakes for Koreans. 2010. 1st rev. Seoul: The Korean Nutrition Society.
19. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980. 92:44–47.
20. Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997. 195:10–19.

22. Burks AW, James JM, Hiegel A, Wilson G, Wheeler JG, Jones SM, Zuerlein N. Atopic dermatitis and food hypersensitivity reactions. J Pediatr. 1998. 132:132–136.

23. Hill DJ, Hosking CS, Zhie CY, Leung R, Baratwidjaja K, Iikura Y, Iyngkaran N, Gonzalez-Andaya A, Wah LB, Hsieh KH. The frequency of food allergy in Australia and Asia. Environ Toxicol Pharmacol. 1997. 4:101–110.

24. Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998. 101:E8.

25. Burks AW, Mallory SB, Williams LW, Shirrell MA. Atopic dermatitis: clinical relevance of food hypersensitivity reactions. J Pediatr. 1988. 113:447–451.

26. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999. 104:S114–S122.

27. Noh G, Ahn HS, Cho NY, Lee S, Oh JW. The clinical significance of food specific IgE/IgG4 in food specific atopic dermatitis. Pediatr Allergy Immunol. 2007. 18:63–70.

28. Van Bever HP, Docx M, Stevens WJ. Food and food additives in severe atopic dermatitis. Allergy. 1989. 44:588–594.

29. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997. 100:444–451.

30. Magnolfi CF, Zani G, Lacava L, Patria MF, Bardare M. Soy allergy in atopic children. Ann Allergy Asthma Immunol. 1996. 77:197–201.

31. Eigenmann PA, Sampson HA. An update on food hypersensitivity. Fundam Clin Immunol. 1994. 2:121–133.
32. Jung JA, Lee JS, Lee SI. Serum specific IgE to egg white, cow's milk, soybean in the children with atopic dermatitis. Pediatr Allergy Respir Dis. 2003. 13:255–262.
33. Lee HK, Pyun BY, Lee SJ. Etiologic allergens, blood eosinophil count and total IgE level according to age and site of skin lesion in atopic dermatitis. Allergy. 1992. 12:70–77.
34. Kim JH, Chung SW, Lim DH, Son BK, Son JA, Lee SI, Cha KE. Food and house dust mite allergens in children with atopic dermatitis. Korean J Allergy. 1997. 17:165–170.
35. Lee HS, Kim JS, Pyun BY. Changes of the prevalence and the allergens of atopic dermatitis in children: In between the year of 1992 and 2002. Pediatr Allergy Respir Dis. 2002. 12:263–271.
36. Oranje AP, Wolkerstorfer A, de Waard-van der Spek FB. Natural course of cow's milk allergy in childhood atopic eczema/dermatitis syndrome. Ann Allergy Asthma Immunol. 2002. 89:52–55.

37. Hammar H. Provocation with cow's milk and cereals in atopic dermatitis. Acta Derm Venereol. 1977. 57:159–163.
38. Hanifin JM. Type I hypersensitivity diseases of the skin: divergent aspects of urticaria and atopic dermatitis. Ann Allergy. 1977. 39:153–160.




 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download