Abstract
A folk prescription consisting of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng has been used in the treatment of diabetes mellitus. The aim of the present investigation was to evaluate the antidiabetic effects of the herb formula extract (HFE) composed of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng in the streptozotocin (STZ)-induced diabetic rats. The HFE was mixed in the food supply of the healthy and STZ-induced diabetic male Sprague-Dawley rats, and its effects on the body weight, water and food intake, hyperglycemia, hypolipidemic and islet structure were studied. The treatment of the rats with STZ for 6 weeks resulted in marasmus, polydipsia, polyphagia, hyperglycemia and hypoinsulinemia. In addition, the diabetic rats showed an apparent decrease in the insulin immunoreactivity and the number of β-cells in the pancreas. The addition of the HFE to the rats' food supply significantly lowered the serum glucose and the serum triglycerides level and preserved the normal histological appearance of the pancreatic islets. These results indicate that the HEF have a strong antidiabetic potential along with the significant hypoglycemic and hypolipidemic effects, which may be applicable in the pharmaceutical industry.
Diabetes mellitus is a hereditary, chronic metabolic disease that has a significant impact on the health and quality of life of the patients [1]. The size of the population who suffers from diabetes worldwide has increased dramatically in the recent years. It had been estimated that the number of people with diabetes would have risen to 221 million by the year 2010, with the figure predicted to climb to as high as 300 million by 2025 [2]. Diabetes mellitus is divided into two major types, the insulin-dependent (IDDM, type 1) and the non-insulin-dependent diabetes mellitus (NIDDM, type 2), with more than 90-95% being of type 2 [3]. Although the two types of diabetes have different pathogenic mechanisms, they are both characterized by hyperglycemia, disturbed carbohydrate, protein and fat metabolism and complications of the eyes, kidneys and nerves. Both types constitute a major cause of morbidity and death [4,5]. The pathologic mechanisms of the disease are mainly from the impaired insulin secretion by the pancreatic β-cells as well as insulin resistance in the target tissues, including the skeletal muscles and the liver, leading to hyperglycemia [6]. In diabetes mellitus, hyperglycaemia generates the reactive oxygen species (ROS), which in turn cause lipid peroxidation and membrane damage, playing an important role in the occurrence of the secondary complications [7]. Hence, the control of the blood glucose level is an effective strategy for preventing or reversing the diabetic complications and improving the quality of life in both type 1 and 2 diabetic patients [8].
Many oral hypoglycemic agents are currently used in the clinical practices, but these synthetic agents are unsatisfactory in terms of preventing secondary complications and adverse side effects of disease [9,10]. Recently, novel herbal medicines have been recommended as novel anti-diabetic treatments for their efficacy in the human clinical trials with minimal side effects [11,12]. The ethnobotanical information on the potential antidiabetic medicinal plants has been reported for approximately 800 plants [13]. However, only a few have been evaluated scientifically regarding their activities.
Generally, in the traditional Asian medicine systems, many herbal drugs are combined into multi-herbal formulas to enhance the effects and reduce toxicity. A folk prescription consisting of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng has been used to traditionally treat diabetes mellitus. Despite the common usage of the folk remedy, there has been no scientific evidence to support the antidiabetic effects of the formula. The purpose of this research was to experimentally assess the anti-diabetic effects of the herb formula extract (HFE) in the streptozotocin (STZ)-induced diabetic rats.
Alnus hirsute, Rosa davurica, Acanthopanax senticosus and Panax schinseng were purchased from a market in Gangwon-do (Republic of Korea). The dried parts of the plants were homogenized to a fine powder. One kilogram of the herbal mixture was composed of Alnus hirsute bark, Rosa davurica root, Acanthopanax senticosus fruit, Acanthopanax senticosus bark and Panax schinseng (w/w/w/w = 7:5:3.5:3.5:1). The herbal mixture was subjected to a boiling-water extraction process with 20 liter of distilled water for 5 h. The suspension was filtered through filter paper and then dried by freezing under high vacuum conditions to obtain the extract of the herbal mixture. The freeze-dried powder of the HFE was blended with the rats' basal diet at the prescribed concentration.
Male Sprague-Dawley rats were purchased from the Orient Animal Inc. (Seoul, Republic of Korea) and housed at room temperature (24 ± 5℃) under relative humidity (50 ± 10%) with a 12 h light / 12 h dark cycle. All animals were allowed free access to a commercial stock diet and water throughout the experiment. Rats weighing 240-290 g were selected for experiments of the STZ-induced hyperglycemia. The research was performed in accordance with the guidelines established by the Kangwon University Institutional Animal Care and Use Committee.
Following overnight fasting (the rats were deprived of food for 16 h but allowed free access to water), diabetes was induced in the rats by an intraperitoneal injection of STZ dissolved in 10 mM cold sodium citrate buffer (pH 4.5, 65 mg/kg of body weight). Control rats were injected with citrate buffer alone. Those rats showing a fasting glucose level of > 280 mg/dl on day 3 (after STZ) were selected for the subsequent study [14]. The data were recorded as day 0 (3 days after injection of STZ) and the subsequent weeks. The diabetic rats were randomized into four groups (Table 1).
During the study period, the food and water intakes were recorded daily while the body weight was measured once a week. The rats were weighed using an electronic balance. The food intake was determined by measuring the difference between the pre-weighed food and the weight of the food remaining in the hopper or spilled every 24 h. The water intake was measured by recording the quantity of water remaining in the feeding bottle.
Six weeks after STZ intoxication, the rats were fasted for 14-15 h, and the blood samples were withdrawn from the vena abdominalis. The blood samples were allowed to clot, and the serum was separated by centrifugation at 3,000 rpm for 15 min. The levels of blood glucose, high density lipoprotein (HDL) cholesterol, cholesterol (CHOL) and triglycerides (TG) were estimated using an auto analyser (Roche Diagnostics GmbH, Penzberg, Germany).
For the microscopic examination, the rats were sacrificed by an overdose of diethyl ether. The sections were immediately excised from each lobe of the pancreatic islet. All samples were embedded in paraffin, cut in sections of 3 µm thickness and stained with hematoxylin and eosin.
For the immunohistological staining of insulin, the pancreas was removed from the animal immediately after sacrificing. The tissue samples were fixed in 4% paraformaldehyde for 48 h and then embedded in paraffin. The sections were incubated with rabbit anti-PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 12 h at 4℃, after which the color was developed using Ni-DAB substrate. After washing with phosphate-buffered saline (PBS), the sections were incubated with biotin-conjugated anti-rabbit IgG for 40 min at room temperature and visualized with ABC reagent. Sections were then rinsed by PBS and incubated with mouse anti-insulin antibody (SantaCruz, rabbit polyclonal IgG, 1:200) at 4℃ for 16 h. Biotinylated goat anti-mouse IgG was used as a secondary antibody. After washing with PBS, the sections were visualized with ABC reagent and the color was developed using Ni-DAB substrate.
Statistical analysis was performed using the SPSS software package. Data analyses were performed by one way analysis of variance (ANOVA) followed by Duncan's multiple range test. All results were expressed as mean ± SD, with six rats in each group. The P-values < 0.05 were considered significant.
The body weight, food intake and water intake of the rats during each experimental period were presented in Fig. 1. The STZ-induced diabetic animals maintained these characteristics during the 6-week investigation period, in contrast to the vehicle-treated diabetic group. There was no significant effect of HFE on the rate of body weight gain in the diabetic rats. However, the treatment with HFE resulted in lower food and water intakes. The treatment of HEF in the normal rats did not alter the body weight at all.
The effects of HFE on the glucose level in the normal and STZ-induced rats are given in Fig. 2. There was a significant elevation in serum glucose in the diabetic control rats compared to the non-diabetic control group (from 160.4 mg/dl to 550.0 mg/dl). An increase in the water intake proportional to the elevation in blood glucose content was observed in the diabetic rats. The increase in the water intake was necessary to depress the elevation of the osmotic pressure due to the increased blood glucose level. However, the serum glucose level in the diabetic rats after the administration of basal diets containing 0.1% HFE was decreased from 372.9 mg/dl (initial value) to 249.2 mg/dl (6 week value). None of the doses of HFE caused any hypoglycemic activity in the normal rats. Fig. 3 revealed the effect of HFE on the levels of CHOL, HDL cholesterol and TG in all of the experimental groups. The serum TG level was significantly higher in the untreated diabetic rats (229.5 mg/dl) compared to the normal rats (98.6 mg/dl), whereas the level of CHOL did not show any significant change. The treatment of the diabetic rats and the non-diabetic rats with the HFE significantly decreased the level of TG and slightly increased the level of HDL cholesterol.
To elucidate the preventative effects of HFE on the STZ-induced diabetes, the pancreatic islets were examined histologically. The pancreatic tissues were obtained 6 weeks after the STZ administration with or without a treatment of the HFE and then subjected to hematoxylin-eosin staining. The non-diabetic rats showed a normal pancreas structure (Fig. 4A), whereas the pancreas of the diabetic control showed degenerative and necrotic changes as well as islet shrinkage (Fig. 4C). The HFE-treated rats showed minimal pathological changes (Fig. 4B). The pancreas of the HFE-treated diabetic rats showed improved islet morphology (Fig. 4D, 4E).
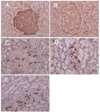
Fig. 5 demonstrates the immunohistochemical effects of the HFE on the pancreas of the experimental rats. In normal and the HFE-treated rats (group A and B), the islets showed a normal structure with an abundance of the insulin-secreting β-cells. Regarding the diabetic group C, a few insulin-secreting β-cells were sporadically scattered in the pancreatic islets of the STZ-induced diabetic rats compared to the control group (Fig. 5A). On the other hand, in the pancreatic islets of group D and E, whose food supply was treated with HEF, a significant increase in the insulin-secreting β cells was observed compared to the untreated diabetic rats.
Diabetes mellitus, the most common endocrine disease, is not a single disease but a group of disorders of varying etiology and pathogenesis [15]. The increase in the number of diabetic patients has motivated scientists to find new agents for a cure. Various plant materials have received an increasing attention and are currently considered to be less toxic with fewer side effects than the synthetic drugs such as biguanides, sulphonylureas, etc [16]. The antihyperglycemic effects of these medical plants are due to their ability to restore the functions of the pancreatic tissues. STZ, a monofunctional nitrosourea derivative, is a broad-spectrum antibiotic derived from Streptomyces achromogenes. Several studies have revealed that STZ possesses diabetogenic properties mediated through pancreatic β-cell destruction, which presents a good model in the search for antidiabetic agents [17]. As such, we investigated the antidiabetic activity of an herbal formula extract composed of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng in the STZ-induced diabetic rats.
The body weight, food intake and water intake of the rats during each experimental period were studied. Induction of diabetes with STZ was related with the reduced rate of body weight gain and the increased food and water intakes. The STZ-induced diabetic animals maintained the characteristics during the 6-week investigation period, in contrast with the vehicle-treated diabetic group. There was no significant effect of the HFE on the rate of body weight gain in the diabetic rats. However, the treatment with the HFE resulted in lower food and water intakes. Treatment of the HEF in the normal rats did not alter the body weight at all. Body weight is one of the general indicators of metabolic regulation for diabetes. Gluconeogenesis in cells is stimulated to compensate for the reduced level of glucose, which results in a decrease in the body weight. However, conflicting results have been reported with there being no alternation between the caffeic acid phenethyl ester treated-rats and the diabetic rats [18].
Diabetes mellitus, characterized by persistent hyperglycemia caused by any of the several possible causes, is the most prominent disease related to a failed blood sugar regulation. There was a significant elevation in the serum glucose in the diabetic control rats compared to the non-diabetic control group. An increase in the water intake proportional to the elevation in the blood glucose content was observed in the diabetic rats. The increase in the water intake was necessary to depress the elevation of the osmotic pressure due to the increased blood glucose level. However, the serum glucose level in the diabetic rats after the administration of the basal diets containing 0.1% HFE was decreased from 372.9 mg/dl to 249.2 mg/dl. This suggests that the blood glucose level was reduced and accompanied by a decreased water intake. The antihyperglycemic effect of the HFE may due to the enhancement of the insulin effect of plasma caused by increased pancreatic secretion of insulin from the insulin-secreting β cells or release of bound insulin.
Diabetes mellitus often involves an abnormally high serum lipid level, which is mainly due to the uninhibited actions of the lipolytic hormones on fat depots [19]. Hypertriglyceridemia and hypercholestrolemia are two major problems in the diabetic patients and are responsible for atherosclerosis and coronary heart disease, the secondary complications of diabetes, although several findings showed that the increased HDL cholesterol content is associated with reduced coronary risk [20,21]. Hyperglycaemia produced a marked increase in the serum triglycerides and the total cholesterol level. Under normal circumstances, insulin induces and activates lipoprotein lipase to hydrolyze triglycerides. Insulin deficiency results in inactivation of these enzymes, thereby causing hypertriglyceridemia [22]. Treatment of diabetic rats and non-diabetic rats with the HFE significantly decreased the level of TG and slightly increased the level of HDL cholesterol. Therefore, it was found that the HFE had not only antihyperglycemic effects but also antihyperlipidemic activity.
Mammalian cells are constantly exposed to ROS as a result of normal metabolic processes occurring during aerobic respiration. However, excessively high levels of free radicals or ROS create oxidative stress, leading to detrimental effects such as lipid peroxidation of the cellular membranes, alteration of the lipid-protein interaction, enzyme inactivation and DNA breakage [23]. STZ is taken up by the pancreatic β-cells via glucose transporter GLUT2. The main cause of STZ-induced β-cell death is via formation of extensive poly (ADP-ribose), depletion of cellular nicotine adenine dinucleotide and ATP, and generation of ROS [24]. Diabetes arises from irreversible destruction of the β-islet cells of the islets of Langerhans in the pancreas, causing deregulation or reduction of insulin [25]. As shown in Fig. 4 and Fig. 5, the pancreas of the HFE-treated diabetic rats showed improved islet morphology; and the pancreatic islets of the HFE-treated diabetic rats showed significant increase in the insulin-secreting β cells. Therefore, the antihyperglycemic and antihyperlipidemic activities of the HFE may be due to the substances present in the HFE which stimulate insulin secretion and/or protect the intact functional β-cells from STZ-induced destruction.
These results clearly indicate that the folk prescription of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng has a high antidiabetic potential along with significant hypoglycemic and hypolipidemic effects and may be applicable in the pharmaceutical industry. However, further studies are necessary define the active mechanism(s) in the complex of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng.
Figures and Tables
Fig. 1
Amount of food intake (A), water intake (B) and variation in average weight (C) of each group. ○: normal rats treated with basal feed diet; □: normal rats treated with feed diet containing 0.01% herb formula extract (HFE); ●: STZ-induced diabetic rats treated with basal feed diet; ■: STZ-induced diabetic rats treated with feed diet containing 0.01% HFE; ▲: STZ-induced diabetic rats treated with feed diet containing 0.1% HFE. Values represent the mean ± SD (n = 6).

Fig. 2
Serum glucose levels in normal and diabetic rats treated with basal feed diet or diet containing herb formula extract (HFE). A: normal rats treated with basal feed diet (Init. GL. = 160.4 ± 9.57 mg/dL); B: normal rats treated with feed diet containing 0.01% HFE (Init. GL. = 156.6 ± 6.68 mg/dL); C: STZ-induced diabetic rats treated with basal feed diet (Init. GL. = 360.6 ± 68.61 mg/dL); D: STZ-induced diabetic rats treated with feed diet containing 0.01% HFE (Init. GL. = 368.3 ± 55.09 mg/dL); E: STZ-induced diabetic rats treated with feed diet containing 0.1% HFE (Init. GL. = 372.9 ± 61.94 mg/dL). Values represent the mean ± SD (n = 6). Values with the same letter are not significantly different by Turkey one way test (P > 0.05). Init. GL, Means the initial serum glucose level of the group.

Fig. 3
Serum lipid content in normal and diabetic rats fed the herb formula extract (HFE) (mg/dL). (■: normal rats treated with basal feed diet; □: normal rats treated with feed diet containing 0.01% HFE; ▥: STZ-induced diabetic rats treated with basal feed diet; ▨: STZ-induced diabetic rats treated with feed diet containing 0.01% HFE; ▤: STZ-induced diabetic rats treated with feed diet containing 0.1% HFE.) Values represent the mean ± SD (n = 6). Values with the same letter are not significantly different by Duncan's multiple range test (P > 0.05).

Fig. 4
H&E staining of rat pancreas (× 400). A: normal rats treated with basal feed diet. B: normal rats treated with feed diet containing 0.01% herb formula extract (HFE). C: STZ-induced diabetic rats treated with basal feed diet. D: STZ-induced diabetic rats treated with feed diet containing 0.01% HFE.E: STZ-induced diabetic rats treated with feed diet containing 0.1% HFE.
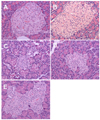
Fig. 5
Insulin immunohistochemistry of rat pancreas (× 400). A: normal rats treated with basal feed diet. B: normal rats treated with feed diet containing 0.01% herb formula extract (HFE). C: STZ-induced diabetic rats treated with basal feed diet. D: STZ-induced diabetic rats treated with feed diet containing 0.01% HFE. E: STZ-induced diabetic rats treated with feed diet containing 0.1% HFE.
References
1. Singh N, Kamath V, Rajini PS. Attenuation of hyperglycemia and associated biochemical parameters in STZ-induced diabetic rats by dietary supplementation of potato peel powder. Clin Chim Acta. 2005. 353:165–175.

2. Hu W, Wang MH. Diarylheotanoid from Alnus hirsuta improves glucose metabolism via insulin signal transduction in human hepatocarcinoma (HepG2) cells. Biotechnol Bioprocess Eng. 2011. 16:120–126.

3. Xing XH, Zhang ZM, Hu XZ, Wu RQ, Xu C. Antidiabetic effects of Artemisia sphaerocephala Krasch. gum, a novel food additive in China, on streptozotocin-induced type 2 diabetic rats. J Ethnopharmacol. 2009. 125:410–416.

6. Baudry A, Leroux L, Jackerott M, Joshi RL. Genetic manipulation of insulin signaling, action and secretion in mice. Insights into glucose homeostasis and pathogenesis of type 2 diabetes. EMBO Rep. 2002. 3:323–328.

7. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a "causal" antioxidant therapy. Diabetes Care. 2003. 26:1589–1596.

8. Chang MS, Oh MS, Kim DR, Jung KJ, Park S, Choi SB, Ko BS, Park SK. Effects of okchun-san, a herbal formulation, on blood glucose levels and body weight in a model of type 2 diabetes. J Ethnopharmacol. 2006. 103:491–495.

9. Vetrichelvan T, Jegadeesan M. Anti-diabetic activity of alcoholic extract of Aerva lanata (L.) Juss. ex Schultes in rats. J Ethnopharmacol. 2002. 80:103–107.

10. Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Arch Intern Med. 2003. 163:1783–1785.

11. Chien SC, Young PH, Hsu YJ, Chen CH, Tien YJ, Shiu SY, Li TH, Yang CW, Marimuthu P, Tsai LF, Yang WC. Antidiabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009. 70:1246–1254.

12. Modak M, Dixit P, Londhe J, Ghaskadbi S, Paul A. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007. 40:163–173.

13. Mentreddy SR. Medicinal plant species with potential antidiabetic properties. J Sci Food Agric. 2007. 87:743–750.

14. Heo SI, Jin YS, Jung MJ, Wang MH. Antidiabetic properties of 2,5-dihydroxy-4,3'-di(beta-D-glucopyranosyloxy)-trans-stilbene from mulberry (Morus bombycis Koidzumi) root in streptozotocin-induced diabetic rats. J Med Food. 2007. 10:602–607.

15. Lebovitz HE. Etiology and pathogenesis of diabetes mellitus. Pediatr Clin North Am. 1984. 31:521–530.

16. Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006. 27:1–93.

17. Dennis JC, Coleman ES, Swyers SE, Moody SW, Wright JC, Judd R, Zhong Q, Morrison EE. Changes in mitotic rate and GFAP expression in the primary olfactory axis of streptozotocin-induced diabetic rats. J Neurocytol. 2005. 34:3–10.

18. Celik S, Erdogan S, Tuzcu M. Caffeic acid phenethyl ester (CAPE) exhibits significant potential as an antidiabetic and liver-protective agent in streptozotocin-induced diabetic rats. Pharmacol Res. 2009. 60:270–276.

19. Pari L, Saravanan G. Antidiabetic effect of Cogent db, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C Toxicol Pharmacol. 2002. 131:19–25.

20. Stirban AO, Tschoepe D. Cardiovascular complications in diabetes: targets and interventions. Diabetes Care. 2008. 31:Suppl 2. S215–S221.
21. Després JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Québec cardiovascular study. Atherosclerosis. 2000. 153:263–272.

22. Badole SL, Bodhankar SL. Antidiabetic activity of cycloart-23-ene-3β, 25-diol (B2) isolated from Pongamia pinnata (L. Pierre) in streptozotocin-nicotinamide induced diabetic mice. Eur J Pharmacol. 2010. 632:103–109.

23. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003. 79:829–843.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download