Abstract
Pumpkins have considerable variation in nutrient contents depending on the cultivation environment, species, or part. In this study, the general chemical compositions and some bioactive components, such as tocopherols, carotenoids, and β-sitosterol, were analyzed in three major species of pumpkin (Cucurbitaceae pepo, C. moschata, and C. maxima) grown in Korea and also in three parts (peel, flesh, and seed) of each pumpkin species. C. maxima had significantly more carbohydrate, protein, fat, and fiber than C. pepo or C. moschata (P < 0.05). The moisture content as well as the amino acid and arginine contents in all parts of the pumpkin was highest in C. pepo. The major fatty acids in the seeds were palmitic, stearic, oleic, and linoleic acids. C. pepo and C. moschata seeds had significantly more γ-tocopherol than C. maxima, whose seeds had the highest β-carotene content. C. pepo seeds had significantly more β-sitosterol than the others. Nutrient compositions differed considerably among the pumpkin species and parts. These results will be useful in updating the nutrient compositions of pumpkin in the Korean food composition database. Additional analyses of various pumpkins grown in different years and in different areas of Korea are needed.
Pumpkins are gourd squashes of the genus Cucurbita and the family Cucurbitaceae. The pumpkin species available include C. pepo (called "Kuksuhobak" in Korean), C. moschata ("neulgeunhobak"), and C. maxima ("danhobak"). These three species are cultivated worldwide and have high production yields [1].
Pumpkins are cooked and consumed in many ways, and most parts of the pumpkin are edible, from the fleshy shell to the seeds. In Korea, pumpkin flesh is consumed in soups and juices, or it is incorporated into various foods, such as rice cakes, candies, and breads. In the US and Canada, pumpkin is a Halloween and Thanksgiving staple. Pumpkin seeds and pumpkin seed oil are also commonly consumed in some countries.
Pumpkins have long been used for traditional medicine in many countries, such as China, Argentina, India, Mexico, Brazil, and Korea, since pumpkin flesh and seeds are rich not only in proteins, antioxidant vitamins, such as carotenoids and tocopherols [2], and minerals, but low in fat and calories. β-carotene reduces skin damage from the sun and acts as an anti-inflammatory agent. α-carotene is thought to slow the aging process, reduce the risk of developing cataracts, and prevent tumor growth. Vitamin E (tocopherols) protects the cell from oxidative damage by preventing the oxidation of unsaturated fatty acids in cell membrane. Pumpkin seeds, often eaten as a snack, are a good source of zinc, polyunsaturated fatty acids [3,4], and phytosterols (e.g. β-sitosterol) [1,5], which can prevent chronic diseases. Recent studies have reported that pumpkin can benefit the treatment of benign prostate hyperplasia, because of its high β-sitosterol content [6-9]. β-Sitosterol has been indicated to reduce blood cholesterol and to decrease risks of certain types of cancers.
The most frequently consumed Cucurbita species in Korea are C. moschata and C. maxima, whereas C. pepo consumption is relatively low. Thus, there is limited research regarding C. pepo in Korea. Other countries, however, including the US and Canada consume more C. pepo than other species. In 2006, the National Rural Living Science Institute in Korea updated their food composition tables [10]. The Korean food composition tables include 4 types of pumpkins (mature pumpkin, young pumpkin, zucchini squash, and sweet pumpkin), mainly C. moschata and C. maxima [10]. Some nutrient contents in C. pepo are also reported, but the amino acid, fatty acid, vitamin E, and carotenoid contents in C. pepo are not available. Currently, there is limited research analyzing the nutrients in C. pepo grown in Korea, and the nutrients in the different parts of each pumpkin species. Because the nutrient composition of pumpkins will differ depending on their origins and cultivation environments [11-15], it may be important to know the nutritional profiles of the various pumpkin species grown in Korea and of the various parts of these pumpkins. Moreover, C. pepo harvest and consumption are gradually increasing in Korea. Therefore, this study determined the general nutrient composition, including amino acids, fatty acids, and specific bioactive nutrients, such as tocoperols, carotenoids, and β-sitosterol, of 3 pumpkin species grown and consumed in Korea (C. pepo, C. moschata, and C. maxima) and 3 different parts (peel, flesh, and seed) of each species.
C. pepo was obtained from a local farm (Gunsan, Korea). C. moschata (Naju, Korea) and C. maxima (Kochang, Korea) were purchased from agricultural product joint markets in Kwangju, Korea. Over 20 pumpkins of each species were purchased. All samples were harvested and collected in the fall of 2008. The samples were divided into 3 parts: peel, flesh, and seed. Samples were freeze-dried, mixed using a hand blender (PHILIPS HR-1372, Koninklijke Philips Electronics N.V., Amsterdam, Netherlands), and stored at -70℃ until analyzed. All samples in this study were analyzed in triplicate.
An amino acid standard solution (AA-S-18) was purchased from Fluka Ltd. (Buchs, Switzerland). A fatty acid 37 component methyl ester mix was obtained from Supelco™ (Bellefonte, PA, USA). α- and γ-tocopherol, β-carotene, β-cryptoxanthin, and β-sitosterol standards were obtained from Sigma Chemical Co. (St Louis, MO, USA).
High-performance liquid chromatography (HPLC) grade hexane (JT Baker, Deventer, Holland), tetrahydrofuran (THF, Acros Organics Co., Geel, Belgium), methanol (JT Baker, Deventer, Holland), and acetonitrile (JT Baker, Deventer, Holland) were used. Triethylamine (Fisher Scientific Ltd., Loughborough, UK), dichloromethane (Acros Organics Co., Geel, Belgium), and N,O-Bis (trimethylsilyl) trifluoroacetamide (BHT, Acros Organics Co.) were purchased. All other reagents used were analytical grade.
Protein was analyzed using the macro-Kjeldahl method (AOAC 984.13) using a Foss Kjeltec 2300 automatic analyzer (Foss Tecator AB, Höganäs, Sweden) [16]. Crude fat was analyzed by AOAC method 945.16 with ether as a solvent [16]. Ash was determined by a muffle furnace set at 550℃ (AOAC 942.05) [16]. Moisture content was determined using AOAC 930.15 oven drying method at 105℃ overnight [16]. Total carbohydrate contents were calculated by 100-(g moisture + g protein + g fat + g ash) [17].
Amino acids were measured in hydrolysates using a Sykam-S433D amino acid analyzer (Sykam GmbH, Fürstenfeldbruck, Germany). Hydrolysates were prepared as described by Moore and Stein [18] and modified by Mohammed and Yagoub [19]. Ninhydrin solution and an eluent buffer (solvent A: pH 3.45 and solvent B: pH 10.85) were delivered simultaneously into a high temperature reactor coil (16 m long) at a flow rate of 0.7 mL/min. The buffer/ninhydrin mixture was heated in the reactor to 130℃ for 2 minutes to accelerate the amino acid reaction with ninhydrin. The reaction products were detected with 570 nm and 440 nm light on a dual channel photometer. The amino acid contents were calculated from the areas of standards obtained from the integrator, and are expressed as percentages.
Dried samples were extracted with chloroform:methanol (2:1, v/v) according to the method of Folch et al. [20]. Solid and non-lipid material were removed, then the solvent was evaporated under nitrogen gas. Fatty acid methyl ester was prepared by methylating the total lipids, as described by Joseph and Ackman [21]. Methyl esters were separated by gas chromatography (GC), (Varian 3400 capillary GC with a flame ionization detector, Varian, Walnut Creek, CA, USA and SP-2560, 100 m × 0.25 mm i.d., Supelco Inc., Bellefonte, PA, USA) under the following conditions. The detector temperature was 280℃, the injection port temperature was 250℃, and the column temperature was 180℃. Carrier gas (hydrogen) flow was 1 mL/min with a nitrogen flow of 30 mL/min. The split ratio was 50:1 and samples (1 µL) were injected in triplicate. To identify each fatty acid, each retention time was compared with the standard (Supelco 37 fatty acid methyl esters).
Tocopherols and carotenoids were extracted from pumpkin seeds using a method modified from Kim et al. [22], and using HPLC (Gilson 351 HPLC system, Gilson, Villiers le Bel, France) with a 151 UV/VIS detector and a C18 column (250 × 4.6 mm i.d., 5 µm, GraceSmart™, Deerfield, USA). The mobile phase was 40 mL of water (containing triethylamine [500 µL] and ammonium acetate [0.4 g]), 60 mL of methanol (containing BHT [1 g L-1]), 800 mL of acetonitrile, and 100 mL THF. The flow rate was 1.0 mL/min, and the column temperature was 24℃. Tocopherols and carotenoids were detected at 297 nm and 450 nm, respectively. Tocopherols and carotenoids were quantified using calibration curves obtained with each standard alone and mixed.
Two grams of pumpkin seeds were hydrolyzed with 6 M HCl as described by Toivo et al. [23]. Dried extracts were saponified as described by Maguire et al. [24]. The hexane layer was dried under nitrogen, redissolved in 200 µL ethanol, and stored at -20℃ for HPLC analysis on a Gilson HPLC system (Gilson, Villiers le Bel, France) with a Luna C8(2) column (250 × 4.6 mm i.d., 5 µm, Phenomenex, Cheshire, UK). The mobile phase was 100% acetonitrile, flow rate was 1.2 mL/min, and the column temperature was 24℃. β-sitosterol was detected at 208 nm using a UV detector.
All statistical analyses were performed using SPSS 15.0 (SPSS, Inc., Chicago, USA). In order to determine the differences in nutrient contents among species, one-way ANOVA tests were performed, followed by post-hoc test (Duncan's multiple range test) to compare means. A P value < 0.05 was considered significant. Data are presented as mean ± standard deviation (SD).
Table 1 shows the chemical compositions each pumpkin species. The contents of The flesh of C. maxima, C. pepo, and C. moschata contained 26.23 ± 0.20 g carbohydrate/kg raw weight, 42.39 ± 0.84 g/kg, and 133.53 ± 1.44 g/kg, respectively. C. maxima had significantly more carbohydrates in the flesh and peel than C. pepo and C. moschata. C. maxima had significantly more protein in the flesh (11.31 ± 0.95 g/kg raw weight) and peel (16.54 ± 2.69 g/kg raw weight) than C. pepo and C. moschata (P < 0.05). C. pepo had significantly more protein in the seeds (308.8 ± 12.01 g/kg raw weight) than C. maxima (274.85 ± 10.04 g/kg raw weight), (P < 0.05). The flesh of C. pepo and C. moschata had a small amount of fat (0.55 ± 0.14 and 0.89 ± 0.11 g/kg raw weight, respectively). The peel of C. pepo and C. moschata had similar amounts of fat (4.71 ± 0.69 and 6.59 ± 0.41 g/kg raw weight, respectively). C. maxima seeds had significantly more fat (524.34 ± 1.32 g/kg raw weight), (P < 0.05) than C. pepo or C. moschata (439.88 ± 2.88 and 456.78 ± 11.66 g/kg raw weight, respectively). The flesh and seeds of C. pepo had significantly lower fiber and ash contents than C. moschata or C. maxima (P < 0.05). All parts of C. pepo had the highest moisture content, and C. maxima the lowest.
The amino acid compositions are presented in Table 2. Except for aspartic acid, the flesh and peel of C. maxima had higher amino acid contents than the two species. In the seeds C. pepo had the highest amino acid concentrations. Pumpkin seeds were contained all 9 essential amino acids. The arginine content in C. pepo seeds (63.99 ± 0.88 mg/kg raw weight) was much higher than in C. moschata (7.03 ± 0.58 mg/kg raw weight) or C. maxima (8.69 ± 0.97 mg/kg raw weight). Glycine was not detected in the flesh of C. pepo, whereas C. moschata and C. maxima contained small amounts (0.05 ± 0.01 and 0.12 ± 0.01 mg/kg raw weight, respectively). Methionine was not detected in the flesh of C. pepo or C. moschata, but C. maxima contained a small amount (0.11 ± 0.00 mg/kg raw weight).
Table 3 shows the fatty acid compositions in pumpkin seeds. Seven kinds of fatty acids in C. pepo, 4 fatty acids in C. moschata, and 10 fatty acids in C. maxima were detected in this study. The seeds were 18.62-20.11% saturated fatty acid, 14.90-32.40% monounsaturated fatty acid (MUFA), and 35.72-56.84% polyunsaturated acids (PUFA). C. pepo and C. moschata seeds contained similar amounts of oleic acid (C. pepo: 32.40 ± 0.56% fat, C. moschata: 31.34 ± 0.12% fat) and linoleic acid (C. pepo: 36.40 ± 0.82% fat, C. moschata: 35.72 ± 0.25% fat), but C. maxima seeds contained more linoleic acid (56.60 ± 0.29% fat) than oleic acid (14.83 ± 0.05% fat). C. maxima had 3-fold higher PUFA than MUFA. The PUFA content in C. maxima was significantly higher than C. pepo and C. moschata (P < 0.05).
The tocopherol and carotenoid concentrations of the pumpkins are presented in Table 4. C. maxima had the highest α-tocopherol content in the peel, but the 3 species did not differ significantly. The α-tocopherol contents in the seeds of C. pepo, C. moschata, and C. maxima were 21.33 ± 3.65, 25.74 ± 0.73, and 20.73 ± 1.33 mg/kg raw weight, respectively. In the flesh, only C. moschata contained γ-tocopherol. C. pepo and C. maxima peels contained γ-tocopherol. The γ-tocopherol contents of the seeds in C. pepo (61.65 ± 17.66 mg/kg raw weight) and C. moschata (66.85 ± 4.90 mg/kg raw weight) were higher than C. maxima seeds (28.70 ± 2.13 mg/kg raw weight), (P < 0.05). The peels in all 3 species had more β-carotene than the other parts. The β-carotene concentration in seeds was the highest in C. maxima (31.40 ± 3.02 mg/kg raw weight). β-Cryptoxanthin was detected in the flesh of only C. maxima, in the peels of all 3 species, and in the seeds of C. pepo and C. maxima.
The β-sitosterol contents and presented in Table 5. C. pepo seeds had significantly more β-sitosterol (383.89 ± 48.15 mg/kg raw weight), (P < 0.05) than C. moschata and C. maxima (277.58 ± 23.48 and 235.16 ± 1.44 mg/kg raw weight, respectively).
The general chemical compositions and select bioactive components, including tocopherols, carotenoids, and β-sitosterol, were analyzed in 3 pumpkin species (C. pepo, C. moschata, and C. maxima) grown in Korea, and also in 3 parts (peel, flesh, and seed) of the pumpkin.
C. maxima had significantly more carbohydrates in the flesh than C. pepo and C. moschata. This high carbohydrate concentration may contribute to the sweet taste of C. maxima. Because of its sweet taste, C. maxima is called "Danhobak" in Korean, "Dan" meaning "sweet" and "hobak" meaning "pumpkin." The C. maxima flesh and peel had significantly more protein than C. pepo or C. moschata. C. pepo seeds had significantly more protein than C. maxima seeds (P < 0.05). We found 20-25% more protein in the C. pepo seeds than reported in other studies [25-27], but 37-44% less protein than reported by Idouraine et al. [28]. We found 43.99-52.43% fat in the seeds, which is higher than the 24.2-45.1% reported for four Cucurbita species (C. moschata, C. maxima, C. pepo, and C. argyrosperma) grown in a common garden of Missouri, USA [15] and the 22-35% reported in African C. pepo [29]. C. pepo had the most moisture in all parts, and C. maxima had the lowest. The moisture contents in the current study were similar to previous reports for C. maxima (87.6%) and C. moschata (92.3%) [30].
The flesh and peel of C. maxima had more amino acids than the other two species. C. pepo seeds generally had more amino acids than C. moschata and C. maxima. Pumpkin seeds contained all 9 essential amino acids. The histidine, leucine, and valine contents were higher than the other essential amino acids. The most significant difference in amino acid levels was for arginine. C. pepo seeds had over 6 times more arginine than C. moschata or C. maxima. The arginine content (63.99 ± 0.88 mg/kg raw weight, 18.81%) of C. pepo seeds was similar to a previous report of 14-18% [28]. The amino acid profile of C. moschata in the current study was consistent with a previous study analyzing C. moschata cultivated in Korea [31].
In the current study, fatty acids were not analyzed in the flesh and peel because the fatty acid content in these parts was expected to be below the level of detection (0.1 g per 100 g edible pumpkin, data from the USDA nutrient database) [32]. Applequist et al. [15] detected stearic, palmitic, oleic, and linoleic acids in C. pepo seeds, while we detected 7 fatty acids in C. pepo, 4 fatty acids in C. moschata, and 10 fatty acids in C. maxima. The major fatty acids were palmitic (C16:0), stearic (C18:0), oleic (C18:1), and linoleic (C18:2) acids. Our results parallel several previous studies reporting that palmitic, stearic, and linoleic acid were the major fatty acids in pumpkin seeds [3,33]. C. maxima seeds contained 3-fold more PUFA than MUFA, which was significantly higher than C. pepo and C. moschata (P < 0.05). The linoleic acid concentration in C. moschata seeds was higher than C. pepo in other studies [15,34]. In our study, the linoleic acid concentration in C. maxima was higher than other species (P < 0.05).
Tocopherols and carotenoids have been suggested to be fat-soluble antioxidants. Antioxidants play an important role in decreasing DNA damage, diminishing lipid peroxidation, maintaining immune function, and inhibiting malignant transformation or proliferation in vitro, which are thought to prevent some diseases [35]. C. maxima had more α-tocopherol in the flesh and peel that the other species; however, the differences were not significant (P > 0.05). γ-Tocopherol was only present in C. moschata flesh, C. pepo and C. maxima peels, and the seeds of all 3 species. The α-tocopherol content (2.31 mg/kg) in C. maxima flesh was much lower than reported in the USDA nutrient database (1.06 mg/100 g edible pumpkin) [32]. The α- and γ-tocopherol levels in pumpkin seeds in this study were lower than reported for 12 pumpkin seed cultivars from the USA [2]. Stevenson et al. [2] reported α-tocopherol and γ-tocopherol contents between 27.1-75.1 mg/kg and 74.9-492.8 mg/kg, respectively. γ-Tocopherol contents in C. pepo and C. moschata seeds were typically 2.5-3.0 times higher than α-tocopherol. α-Tocopherol has the greatest bioavailability, however, γ-tocopherol may have higher antioxidant activities [36,37]. Whang et al. [38] reported that β-carotene contents in the flesh and peel of C. moschata cultivated in Korea were similar. In this study, the β-carotene contents in the peels of 3 species were 5-15 folds higher than in the flesh.
Each pumpkin part in this study contained a significant amount of antioxidants, tocopherols, and carotenoids. Therefore, pumpkin potentially has antioxidant activity, which might be important for pre-diabetics, diabetics, and patients with vascular injury [39]. Administering pumpkin extract significantly increased superoxide dismutase and glutathione peroxidase activities in mouse liver [40]. Diets high in pumpkin seeds have been associated with lower risks of gastric, breast, lung, and colorectal cancers [41]. The carotenoids in pumpkin flesh might prevent prostate cancer [42]. In addition to fat-soluble antioxidants (tocopherols and carotenoids), C. maxima had 16 mg vitamin C per 100 g raw pumpkin [10]. Vitamin C is a strong water-soluble antioxidant that protects cells and cellular components from free radicals by donating electrons, and regenerating other antioxidants, such as vitamin E (tocopherols) [43]. Therefore, high pumpkin intake has various benefits to improve overall health. Currently, pumpkins are consumed as vegetables and medicines in many countries, such as China, Argentina, India, Mexico, Brazil, the US, and Korea. It is commonly used to prevent diabetes and eliminate intestinal parasites [44]. In Korea, pumpkins have been used traditionally to relieve edema during pregnancy and after delivery. Among the 3 species in this study, extracts of C. maxima and C. moschata flesh are frequently used as a medicine in Korea [45]. Although the peels are usually discarded in Korea, they contain much more tocopherols and carotenoids than the flesh, thus they may have a domestic use as medicine.
β-Sitosterol is a phytosterol, which are integral components of plant cell membranes, and are abundant in vegetable oils, nuts, seeds, and grains [46]. Phytosterols can lower both total serum cholesterol and LDL-cholesterol in humans by inhibiting the absorption of dietary cholesterol [47], and can prevent cancer [48]. Recently, plant sterols have been proposed to have other positive health effects [49]. β-Sitosterol especially is considered a treatment for benign prostatic hyperplasia [8]. C. pepo seeds had significantly more β-sitosterol (383.89 ± 48.15 mg/kg raw weight), (P < 0.05) than C. moschata and C. maxima (277.58 ± 23.48 and 235.16 ± 1.44 mg/kg raw weight, respectively). The β-sitosterol content in C. pepo in this study was similar to barley (381 mg/kg) and maize (341 mg/kg) [5]. Ryan et al. [5] reported that the β-sitosterol content in pumpkin seeds was 249 mg/kg, which is similar to C. moschata and C. maxima in our study. The pumpkin seeds in this study (cultivated in Korea) had more β-sitosterol than pumpkin seed oils cultivated in the USA [1]. The high β-sitosterol contents in this study may result from the cultivars, growing seasons, and planting locations, which maximize the phytosterol concentrations in plants [1]. β-Sitosterol might have broad biological effects including lowering cholesterol, estrogenic activity, and anticarcinogenic activity [48,49]. Therefore, pumpkin seeds highly containing β-sitosterol would help maintain human health.
In summary, the amino acid contents were higher in the seeds than the flesh or peel. Amino acid contents in C. pepo seeds were higher than C. moschata and C. maxima. The major fatty acids were palmitic, stearic, oleic, and linoleic acid. The α-tocopherol concentration was highest in C. pepo peel, but the 3 species did not differ significantly. γ-Tocopherol was detected in the seeds of all species. There was no significant difference in β-carotene contents of the flesh and peel. The β-carotene content in seeds was highest in C. maxima. C. pepo seeds had significantly more β-sitosterol than C. moschata and C. maxima. This study should help updating nutrient compositions in the Korean food composition database, as well as estimate more accurate dietary intake and nutrient adequacies from food consumption surveys in Korea. Further research on the nutrient composition of pumpkins is needed, including analyses of various pumpkins grown in different years and different areas of Korea.
Figures and Tables
Table 1
Chemical compositions (g/kg raw weight) of pumpkins (Cucurbitaceae) by species and by part1)

Table 2
Amino acids concentrations (mg/kg raw weight) in pumpkins (Cucurbitaceae) by species and by part1)
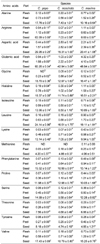
Table 3
Fatty acid concentrations (% fat) in pumpkin seeds (Cucurbitaceae) by species1)

1) Results are expressed as a % of the total fatty acid fraction. Values are mean ± SD. Different superscript letters within a row indicate significant differences by Duncan's multiple range test (P < 0.05).
ND, not detected; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid
References
1. Phillips KM, Ruggio DM, Ashraf-Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J Agric Food Chem. 2005. 53:9436–9445.

2. Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T, Inglett GE. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem. 2007. 55:4005–4013.

3. Glew RH, Glew RS, Chuang LT, Huang YS, Millson M, Constans D, Vanderjagt DJ. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods Hum Nutr. 2006. 61:51–56.
4. Sabudak T. Fatty acid composition of seed and leaf oils of pumpkin, walnut, almond, maize, sunflower and melon. Chem Nat Compd. 2007. 43:465–467.

5. Ryan E, Galvin K, O'Connor TP, Maguire AR, O'Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum Nutr. 2007. 62:85–91.

6. Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. Br J Urol. 1990. 66:639–641.

7. Dvorkin L, Song KY. Herbs for benign prostatic hyperplasia. Ann Pharmacother. 2002. 36:1443–1452.

8. Gossell-Williams M, Davis A, O'Connor N. Inhibition of testosterone-induced hyperplasia of the prostate of Sprague-Dawley rats by pumpkin seed oil. J Med Food. 2006. 9:284–286.

9. Tsai YS, Tong YC, Cheng JT, Lee CH, Yang FS, Lee HY. Pumpkin seed oil and phytosterol-F can block testosterone/prazosin-induced prostate growth in rats. Urol Int. 2006. 77:269–274.

10. National Rural Living Science Institute. Food Composition Table. 2006. 7th revision. Suwon: Rural Development Administration.
11. Park YK, Cha HS, Park MW, Kang YH, Seog HM. Chemical components in different parts of pumpkin. J Korean Soc Food Sci Nutr. 1997. 26:639–646.
12. Jang SM, Park NY, Lee JB, Ahn H. The comparison of food constituent in different parts of pumpkin. J Korean Soc Food Sci Nutr. 2001. 30:1038–1040.
13. Heo SJ, Kim JH, Kim JK, Moon KD. The comparision of food constituents in pumpkin and sweet-pumpkin. Korean J Food Cult. 1998. 13:91–96.
14. Achu MB, Fokou E, Tchiégang C, Fotso M, Tchouanguep FM. Nutritive value of some Cucurbitaceae oil seeds from different regions in Cameroon. Afr J Biotechnol. 2005. 4:1329–1334.
15. Applequist WL, Avula B, Schaneberg BT, Wang YH, Khan IA. Comparative fatty acid content of seeds of four Cucurbita species grown in a common (shared) garden. J Food Compost Anal. 2006. 19:606–611.

16. AOAC International. Official Methods of Analysis of AOAC International. 2000. 17th ed. Arlington: AOAC International.
17. Agriculture Handbook No. 8, Composition of Foods. 1975. Washington D.C.: United States Department of Agriculture;159–165.
18. Moore S, Stein WH. Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol. 1963. 6:819–831.
19. Mohammed MA, Yagoub AA. Fururndu, from fermented/sprouted roselle (Hibiscus sabdariffa L.) seed: investigation on chemical composition, antinutritional factors, HCl-extractability of minerals, amino acids composition, in vitro protein digestibility and microbial growth. Res J Agric Biol Sci. 2007. 3:876–885.
20. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226:497–509.

21. Joseph JD, Ackman RG. Capillary column gas chromatography method for analysis of encapsulated fish oil and fish oil ethyl esters: collaborative study. J AOAC Int. 1992. 75:488–506.

22. Kim YN, Giraud DW, Driskell JA. Tocopherol and carotenoid contents of selected Korean fruits and vegetables. J Food Compost Anal. 2007. 20:458–465.

23. Toivo J, Phillips K, Lampi AM, Piironen V. Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols. J Food Compost Anal. 2001. 14:631–643.

24. Maguire LS, O'Sullivan SM, Galvin K, O'Connor TP, O'Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004. 55:171–178.

25. Scheerens JC, Ralowicz AE, McGriff TL, Bee KA, Nelson JM, Gathman AC. Phenotypic variation of agronomic traits among coyote gourd accessions and their progeny. Econ Bot. 1991. 45:365–378.

26. Lazos ES. Certain functional properties of defatted pumpkin seed flour. Plant Foods Hum Nutr. 1992. 42:257–273.

27. Vasconcellos JA, Bemis WP, Berry JM. Weber CW. The buffalo gourd, Cucurbita foetidissima HBK, as a source of edible oil. J Am Oil Chem Soc. 1981. 9:55–68.
28. Idouraine A, Kohlhepp EA, Weber CW. Nutrient constituents from eight lines of naked seed squash (Cucurbita pepo L.). J Agric Food Chem. 1996. 44:721–724.

29. Younis YM, Ghirmay S, al-Shihry SS. African Cucurbita pepo L.: properties of seed and variability in fatty acid composition of seed oil. Phytochemistry. 2000. 54:71–75.

30. Kim SR, Ha TY, Song HN, Kim YS, Park YK. Comparison of nutritional composition and antioxidative activity for kabocha squash and pumpkin. Korean J Food Sci Technol. 2005. 37:171–177.
31. Youn SJ, Jun HJ, Kang SC. Content analyses of fiber, protein and amino acids of fully ripe fruits of Korea native squash, Cucurbita moschata poir. J Korean Soc Appl Biol Chem. 2004. 47:403–408.
32. USDA national nutrient database for standard reference, release 21. U.S. Department of Agriculture, Agricultural Research Service [Internet]. 2008. cited 2010 March 30. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=18880.
33. Tsaknis J, Lalas S, Lazos ES. Characterization of crude and purified pumpkin seed oil. Grasas Y Aceites. 1997. 48:267–272.

34. Al-Khalifa AS. Physicochemical characteristics, fatty acid composition, and lipoxygenase activity of crude pumpkin and melon seed oils. J Agric Food Chem. 1996. 44:964–966.

35. Gropper SS, Smith JL, Groff JL. Advanced Nutrition and Human Metabolism. 2005. 4th ed. Belmont: Thomson Wadsworth Publishing Co.;381–405.
36. Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab. 2004. 48:169–188.
37. Saldeen K, Saldeen T. Importance of tocopherols beyond α-tocopherol: evidence from animal and human studies. Nutr Res. 2005. 25:877–889.

38. Whang HJ, Park YK, Seog HM. Carotenoid pigment of pumpkin cultivated in Korea. Korean J Food Nutr. 1999. 12:508–512.
39. Yadav M, Jain S, Tomar R, Prasad GB, Yadav H. Medicinal and biological potential of pumpkin: an updated review. Nutr Res Rev. 2010. 23:184–190.

40. Chang D, Pan Hz, Jin JW, Yu Cl, Cao J. Effect of pumpkin distillable subject on lipid peroxidation and the activity of antioxidative enzyme induced by Plumbum in mouse. Chin J Clin Rehabil. 2004. 8:4378–4379.
41. Huang XE, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J, Takezaki T, Tajima K. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac J Cancer Prev. 2004. 5:419–427.
42. Jian L, Du CJ, Lee AH, Binns CW. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. 2005. 113:1010–1014.

43. Keith RE. Driskell JA, Wolinsky I, editors. Ascorbic acid. Sports Nutrition: Vitamins and Trace Elements. 2006. Boca Raton, FL: CRC Press;Chapter 2.

44. Caili F, Huan S, Quanhong L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum Nutr. 2006. 61:73–80.

45. Jang SM, Park NY, Lee JB, Ahn H. The comparison of food constituent in different parts of pumpkin. J Korean Soc Food Sci Nutr. 2001. 30:1038–1040.
46. Weihrauch JL, Gardner JM. Sterol content of foods of plant origin. J Am Diet Assoc. 1978. 73:39–47.

47. Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000. 80:939–966.

48. Raicht RF, Cohen BI, Fazzini EP, Sarwal AN, Takahashi M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980. 40:403–405.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download