Abstract
This study investigated the effects of inorganic sulfur on metastasis in MDA-MB-231 human breast cancer cells. MDA-MB-231 cells were cultured in the absence or presence of various concentrations (12.5, 25, or 50 µmol/L) of inorganic sulfur. Cell motility, invasion, and the activity and mRNA expression of matrix metalloproteases (MMPs) were examined. Numbers of viable MDA-MB-231 cells did not differ by inorganic sulfur treatment from 0 to 50 µmol/L within 48 h. Inorganic sulfur significantly decreased cell motility and invasion in the MDA-MB-231 cells in a dose-dependent manner (P < 0.05), as determined using a Boyden chamber assay and a Matrigel chamber. The activities of MMP-2 and MMP-9 were significantly reduced by inorganic sulfur in a dose-dependent manner (P < 0.05). The inorganic sulfur also significantly inhibited MMP-2 and MMP-9 expression in the cells (P < 0.05). These data suggest that inorganic sulfur can suppress cancer cell motility and invasion by inhibiting MMP-2 and MMP-9 activity and gene expression in MDA-MB-231 cells.
The World Health Organization (WHO) has estimated that from 2007 to 2030, the number of cancer patients will increase from 11.3 million to 15.5 million, and death caused by cancer will increase by 45% from 7.9 million to 11.5 million, indicating that cancer is one of the major causes of death worldwide [1]. The number of cancer patients is increasing in Korea as well; it is reported that -120 thousand individuals became cancer patients in 2008, with > 550 thousand already being established patients [2].
In the case of breast cancer, Korea had 11,439 new patients in 2008 (both male and female) and it was ranked in 6th in the occurrence rate of new patients [3]. For female patients only, it was ranked 2nd in occurrence rate, and 1st in the number of treated patients (National Health Insurance, all rights reserved) [2].
Breast cancer is divided into invasive and non-invasive categories according to its metastatic behavior, and > 75% of breast cancer is invasive. Thus, although it is important to prevent the occurrence of breast cancer per se, preventing metastasis after breast cancer has occurred can be a crucial way to reduce the resulting death rate. In general, the mechanism of tumor metastasis is as follows. Tumor cells migrate from primary tumors and attach to basement membranes, whose extracellular-matrix (ECM) made of collagen, is degraded by matrix metalloproteases (MMPs), causing invasion into proximal tissues [4-6]. The tumor cells then enter the circulation system including blood / lymphatic vessels, and circulate through and penetrate vessels of certain tissues to cause metastatic cancer [7-9]. More than 90% of breast cancer occurs at Lactiferous ducts, which have widely distributed lymphatic vessels through which cancer can easily metastasize [10].
Sulfur is a chemical element with an atomic number of 16 (MW 32.064) and is denoted by S. It is insoluble in water, but soluble in alcohol, benzene, and ether [11]. Chemicals containing sulfur are usually involved in redox (oxidation-reduction) reactions in the body [12]. The human body can ingest sulfur from water and food, mainly as organic sulfur from sulfur-containing amino acids (SAAs) in foods [13]. However, it is reported that although a sufficient amount of sulfur-containing amino acids are ingested, sulfate deficiency symptoms can occur if the amount of dietary sulfate is insufficient, and thus a supply of dietary sulfate is essential for mice [14]. It is also reported that dietary sulfate for animals can supply the sulfur needed to form cysteine, and thus affect the function of bioactive materials that have cysteine as their precursor [15]. Although inorganic sulfur is thought to play an independent physiological role, little research has been done on this topic.
Toxicity by inorganic sulfur is uncommon and is usually limited to the skin [4]. No toxicity was reported from oral administration of 0.17 mg/kg to humans, or in other animal tests with rabbits, rats, guinea pigs, and dogs [5].
The anti-cancer effects of organic sulfur contained in foods have been reported [16-18], but those of inorganic sulfur have rarely been examined. Thus, in this study, we explored whether inorganic sulfur can affect the motility and invasion of human MDA-MB-231 breast cancer cells, as well as MMP-2 and MMP-9 activity and mRNA expression to affect tumor metastasis.
Inorganic sulfur powder of ≥ 99% purity was obtained from Sulfon PS Inc. (Seoul, Korea). The inorganic sulfur powder was dissolved in methyl alcohol (Aldrich, 320390, USA) at a 5 mM concentration and stored at -20℃. Dulbecco's Modified Eagle's medium and Ham's F12 Nutrient Mixture (DMEM/F12), as well as streptomycin and penicillin were obtained from Gibco/BRL (Grand-Island, NY, USA). RIA-grade bovine serum albumin (BSA), transferrin, and other reagents were purchased from Sigma (St. Louis, MO, USA).
MDA-MB-231 human breast cancer cells were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were maintained in DMEM/F12 containing 100 ml/L of fetal bovine serum (FBS) with 100,000 U/L of penicillin and 100 mg/L of streptomycin. The medium was replaced every 2-3 days. To examine the effects of inorganic sulfur on breast cancer cell proliferation, MDA-MB-231 cells were plated in 24-well plates at a density of 2.5 × 104 cells/mL in DMEM/F12 supplemented with 10% FBS. After 48 h of incubation, the monolayers were serum-starved with DMEM/F12 supplemented with 5 µg/mL transferrin, 5 ng/mL selenium, and 1 mg/mL bovine serum albumin for 24 h. After serum starvation, the monolayers were incubated in serum free medium (SFM) with 0, 12.5, 25, or 50 µmol/L inorganic sulfur. Viable cell numbers were estimated at 0 and 48 h after the cells were exposed to inorganic sulfur by using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described [19]. The experiments were performed independently 3 times.
The Boyden chamber motility assay was performed as previously described [20]. PVPF filters (8 µm diameter pore size) were coated with a 0.01% gelatin solution for 16 h at room temperature. MDA-MB-231 cells resuspended in SFM in the absence or presence of various concentrations (12.5, 25, or 50 µmol/L) of inorganic sulfur were carefully transferred into the upper chamber. The lower chamber was filled with 10% FBS medium to attract the cells. The Boyden chamber was incubated at 37℃ with 5% CO2 for 8 h. After gently removing the filter from the chamber, the cells on the upper chamber side of the filter were removed by wiping the filter with paper. The filter was stained with Diff-Quick stain solution (Dade Behring, Network, NJ, USA) and the cells on the lower surface of the filter were fixed onto a glass slide. The cells in 5 randomly selected microscopic fields (× 400) of the lower slide were then counted. The experiments were performed independently 3 times.
The invasion assay was performed as previously described [21]. Wells of a Matrigel chamber (BD Bioscience, MA, USA) were filled with SFM and adapted at room temperature. MDA-MB-231 cells resuspended in SFM with the absence or presence of various concentrations (12.5, 25, or 50 µmol/L) of inorganic sulfur were carefully transferred into the upper chamber. The lower chamber was filled with 10% FBS medium to attract the cells. The Matrigel chambers were incubated for 12 h at 37℃ with 5% CO2. Then, the cells on the upper surfaces of the filters were removed by wiping the filter with paper. The filters were stained with Diff-Quick stain solution and the cells on the lower surface of the filter were fixed onto a glass slide. Cells in 5 randomly selected microscopic fields (× 400) of the lower slide were counted. The experiments were performed independently 3 times.
MMP activity was investigated as previously described [22]. The cells were seeded into 6-well plates at 1 × 103 cells/mL and incubated in a medium containing 10% FBS for 48 h. The monolayers were incubated in the presence of various concentrations of inorganic sulfur (0, 12.5, 25, 50 µmol/L) for 24 h. The supernatants were collected and concentrated 10-fold in Centricon centrifugal filter devices (Milipore, Bedford, MA, USA), after which the MMP activity in the supernatants was investigated using gelatin zymography. Each supernatant was mixed with 2x sample buffer (lnvitrogen), and zymography was performed using gels (10% polyacrylamide, 1% gelatin). The MMP activities were visualized by staining with Coomassie blue. The experiments were performed independently 3 times.
MMP mRNA levels in the culture medium were determined as previously described [22]. Total RNA was isolated using TRI-reagent (Sigma), and cDNA was synthesized using 2 µg of total RNA with SuperScript II reverse transcriptase (lnvitrogen). For amplification of the cDNA, primers for MMP-2 (upstream primer, 5'-CAGGCTCTTCTCCTTTCSCAAC-3'; downstream primer, 5'-AAGCCACGGCTTGGTTTTCCTC-3') and MMP-9 (upstream primer, 5'-TGGGCTACGTGACCTATGACCAT-3'; downstream primer, 5'-GCCCAGCCCACCTCCACTCCTC-3'; annealing at 55℃ for 1 min with 35 cycles) were used. The polymerase chain reaction (PCR) products were separated on a 1% agarose gel and stained with ethidium bromide. The bands corresponding to each specific PCR product were quantified by densitometric scanning of the exposed film using the Bio-profile Bio-IL application (Vilber-Lourmat).
Statistical analyses were performed using the Statistical Analysis System software (SAS Institute, Cary, NC, USA). The data were expressed as means with standard errors and analyzed via analysis of variance (ANOVA). Statistically significant differences among the means of groups were tested at α = 0.05 using Duncan's multiple range test.
To examine the effects of inorganic sulfur on MDA-MB-231 cell proliferation, cells in a monolayer culture were incubated in SFM with 0, 12.5, 25, or 50 µmol/L inorganic sulfur. Numbers of viable MDA-MB-231 cells did not differ by inorganic sulfur treatment from 0 to 50 µmol/L within 48 h (Fig. 1). To show that the anti-metastatic effect of inorganic sulfur was independent of a decrease in cell proliferation, the inorganic sulfur treatment time did not exceed 48 h in this study.
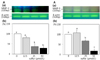
To determine the effects of inorganic sulfur on MMP-2 and MMP-9, which degrade ECM allowing the metastasis of malignant tumors, their activities were tested. Here, the induced activity of MMP-2 and MMP-9 was significantly reduced by inorganic sulfur in a dose-dependent manner (Fig. 4) (P < 0.05).
We performed RT-PCR analysis to determine whether inorganic sulfur regulates MMP-2 and MMP-9 mRNA expression. As shown in Fig. 5, inorganic sulfur significantly inhibited MMP-2 and MMP-9 mRNA expression in the cells (P < 0.05).
Because > 90% of breast cancer occurs at Lactiferous ducts which have widely distributed lymphatic vessels through which the cancer can easily metastasize [10], research on dietary components that can prevent metastasis has great significance. Sulfur is a nutrient whose composition in the body is the seventh highest [13], and research on organic sulfur, such as that contained in cruciferous vegetables, has been performed with regard to its relevance to cancer [16-18]. On the other hand, few studies have focused on inorganic sulfur. Thus, in the present study, we attempted to explore the nutritional and physiological functions of inorganic sulfur by studying its effects on breast cancer metastasis.
When MDA-MB-231 cells were treated with inorganic sulfur, no effects were observed until 48 h of incubation. However, after 72 h of incubation, it was observed from previous tests that cell proliferation was suppressed as the concentration of inorganic sulfur increased (data now shown). In this study, we did not exceed 48 h of inorganic sulfur treatment in order to show that the effects of inorganic sulfur on the motility or invasion of tumor cells were independent from the suppression of cell proliferation.
As a consequence, treatment with inorganic sulfur significantly suppressed tumor motility and invasion in a Boyden chamber and invasion assays at a concentration > 25 µmol/L. Wu et al. treated L9981 lung cancer cells with sulfur-containing benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC), and reported that the motility of BITC and PEITC were decreased to 11.1% and 19.4% (compared to control) after 24 h, of incubation, and to 8.1% and 16.5% after 30 h of incubation, respectively [23]. Ho et al. [24] treated AGS gastric cancer cells with BITC (0, 0.25, or 0.5 µmol/L) in a Boyden chamber motility assay, and reported that tumor cell motility decreased by 42% and 43% for 0.25 and 0.5 µmol/L after 24 h of incubation, respectively, and 37% and 44% after 48 h of incubation, respectively. They concluded that the decrease in tumor motility by organic sulfur was due to a decrease in the ERK signal pathway. Furthermore, Hwang and Lee [25] treated SK-Hep1 human hepatoma cells with allyl isothiocyanates (AITC) and N-acetylcysteine conjugate isothiocyanates (NAC-AITC) at 0-5 µmol/L to explore their effects on tumor cell invasion. As a result, they reported that AITC and NAC-AITC significantly decreased tumor cell invasion at the concentration of 1 µmol/L, and at 5 µmol/L, tumor invasion was decreased by 60% and 53% for AITC and NAC-AITC, respectively. According to the above data, sulfur-containing organics have been reported to decrease the motility and invasion of tumor cells, and it was found in this study that inorganic sulfur has the same effect.
Tumor metastasis is the major cause of death for cancer patients and involves the activation and migration of ECM-degrading enzymes [26]. Consisting of collagen, ECM is degraded by MMPs, and tumor cells will then metastasize to proximal blood vessels [5,6]. Enzymes that can degrade cell membranes and cause the invasion and metastasis of tumor cells include MMPs, serine proteases, cysteine proteases, and aspartyl protenases; among these, MMPs are Zn-dependent peptides that can degrade ECM and play a role in wound healing, tumor invasion, metastasis, and angiogenesis [5,27,28]. MMPs are classified into gelatinases, collagenases, stromelysins, and membrane type MMPs according to enzyme characteristics [29]. High expressions of MMP-2 and MMP-9 that have characteristics of gelatinases are closely related to the metastasis of breast cancer [6,30-33]. Davies et al. [34] reported that the activation of MMP-2 and MMP-9 increased in breast cancer.
In this study, treatment of MDA-MB-231 cells with inorganic sulfur significantly decreased the activation of MMP-2 and MMP-9 and their mRNA expression. Hwang and Lee [25] reported that when SK-Hep1 hepatoma cells were treated with AITC and NAC-AITC, both decreased activation of MMP-2 and MMP-9, but AITC had a greater effect. They also reported that mRNA expression of MMP-2 was significantly decreased by both AITC and NAC-AITC, but that of MMP-9 was decreased by only NAC-AITC.
In conclusion, treatment with inorganic sulfur can decrease the motility and invasion of tumor cells without affecting their proliferation, which is considered to be due to MMP-2 and MMP-9 activation and the suppression of their mRNA expression. In the future, more research on the mechanism of inorganic sulfur's affect on metastasis is necessary.
Figures and Tables
Fig. 1
Effect of inorganic sulfur on cell proliferation in MDA-MB-231 cells. MDA-MB-231 cells were plated at a density of 2.5 × 104 cells/mL in 24 well plates with DMEM/F12 supplemented with 10% FBS for 48 h. The monolayers were serum-starved with DMEM/F12 supplemented with 5 µg/mL transferrin, 5 ng/mL selenium, and 1 mg/mL bovine serum albumin for 24 h. After serum starvation, the monolayer were incubated in serum free medium with 0, 12.5, 25, or 50 µmol/L inorganic sulfur for 24 h. Viable cell numbers were estimated by the MTT assay. Each bar represents the mean ± SE from 3 independent experiments. Significant differences (P < 0.05) among groups are indicated by different letters above each bar.

Fig. 2
Effect of inorganic sulfur on cell motility in MDA-MB-231 cells. Cells were cultured in the presence of various concentrations of inorganic sulfur (0, 12.5, 25, or 50 µmol/L) for 8 h with a Boyden chamber. A) Microphotography of cells treated with inorganic sulfur in cell migration assay. B) Quantitative analysis of cell motility assay. The number of migrated cells was expressed as a percentage of the control (without inorganic sulfur). Each bar represents the mean ± SE from 3 independent experiments. Significant differences (P < 0.05) among groups are indicated by different letters above each bar.

Fig. 3
Effects of inorganic sulfur on invasion in MDA-MB-231 cells. Cells were cultured in the presence of various concentrations of inorganic sulfur (0, 12.5, 25, 50 µmol/L) for 12 h in an invasion chamber. A) Microphotography of cells treated with inorganic sulfur in cell migration assay. B) Quantitative analysis of cell motility assay. The number of migrated cells was expressed as a percentage of the control (without inorganic sulfur). Each bar represents the mean ± SE from 3 independent experiments. Significant differences (P < 0.05) among groups are indicated by different letters above each bar.

Fig. 4
Effects of inorganic sulfur on MMP-2 and MMP-9 activity in MDA-MB-231 cells. Cells were plated in 6 well plates at a density 1 × 103 cell/mL in DMEM/F12 supplemented with 10% FBS. After 48 h of incubation, the monolayers were incubated in the presence of various concentrations of inorganic sulfur (0, 12.5, 25, 50 µmol/L) for 24 h. The medium was collected and concentrated for zymography. A-a) Photographs of MMP-2, A-b) Quantitative analysis of the bands. B-a) Photographs of MMP-9 bands, B-b) Quantitative analysis of the bands. The enzyme activity was expressed as a percentage of the control (without inorganic sulfur). Each bar represents the mean ± SE of 3 independent experiments. Significant differences (P < 0.05) among groups are indicated by different letters above each bar.

Fig. 5
Effect of inorganic sulfur on MMP-2 and MMP-9 mRNA expression in MDA-MB-231 cells. Cells were cultured in the presence of various concentrations of inorganic sulfur (0, 12.5, 25, or 50 µmol/L) for 24 h. Total RNA was isolated and RT-PCR was performed. Photographs of the ethidium bromide-stained gels, which are representative of 3 independent experiments, are shown. A-a) Photographs of the MMP-2 bands, A-b) Quantitative analysis of the bands. B-a) Photographs of the MMP-9 bands, B-b) Quantitative analysis of the bands. The mRNA expression of the enzymes was expressed as a percentage of the control (without inorganic sulfur). Each bar represents the mean ± SE, and significant differences (P < 0.05) among groups are indicated by different letters above each bar.
References
1. Are the number of cancer cases increasing or decreasing in the world? World Health Organization [Internet]. cited 2010 June 3. Available from: http://www.who.int/features/qa/15/en/print.html.
2. Analysis of cancer patients in 2008. National Health Insurance [Internet]. cited 2010 June 5. Available from: http://www.nhic.or.kr/cms/board/board/Board.jsp?act=VIEW&communityKey=B0070&boardId=20108.
3. Types of breast cancer. National Cancer Center [Internet]. cited 2010 June 10. Available from: http://www.cancer.go.kr/cms/cancer/cancer_is/02/1191227_1751.html.
4. Yip D, Ahmad A, Karapetis CS, Hawkins CA, Harper PG. Matrix metalloproteinase inhibitors: applications in oncology. Invest New Drugs. 1999. 17:387–399.
5. Duffy MJ. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992. 10:145–155.

6. Iwata H, Kobayashi S, Iwase H, Masaoka A, Fujimoto N, Okada Y. Production of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human breast carcinomas. Jpn J Cancer Res. 1996. 87:602–611.

7. Kim KW. Tumor metastasis and angiogenesis. J Life Sci. 1994. 4:19–29.
8. Okegawa T, Pong RC, Li Y, Hsieh JT. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim Pol. 2004. 51:445–457.

9. Sin DI, Park JB, Park KK, Cho CH, Oh HK, Choi CH, Cho HJ, Jang YC. Inhibitory effects of type IV collagenase by disulfiram. J Life Sci. 2006. 16:964–971.

10. Structure of breast cancer. National Cancer Center [Internet]. cited 2010 June 10. Available from: http://www.cancer.go.kr/cms/cancer/cancer_is/01/1190409_1221.html.
11. The Merck Index. Sulfur. 2001. 13th ed. Merck & Co., INC.
12. Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002. 7:22–44.
13. Ingenbleek Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J Nutr. 2006. 136:1641S–1651S.

14. Michels FG, Smith JT. A comparison of the utilization of organic and inorganic sulfur by the rat. J Nutr. 1965. 87:217–220.

15. Sasse CE, Baker DH. Sulfur utilization by the chick with emphasis on the effect of inorganic sulfate on the cystine-methionine interrelationship. J Nutr. 1974. 104:244–251.

16. Pappa A, Franco R, Schoneveld O, Galanis A, Sandaltzopoulos R, Panayiotidis MI. Sulfur-containing compounds in protecting against oxidant-mediated lung diseases. Curr Med Chem. 2007. 14:2590–2596.

17. Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000. 60:1426–1433.
18. Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004. 25:83–90.

19. Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid down regulates insulin-like growth factor-1 receptor levels in HT-29 human colon cancer cells. J Nutr. 2003. 133:2675–2681.

20. Soel SM, Choi OS, Bang MH, Yoon Park JH, Kim WK. Influence of conjugated linoleic acid isomers on the metastasis of colon cancer cells in vitro and in vivo. J Nutr Biochem. 2007. 18:650–657.

21. Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008. 19:313–319.

22. Na MH, Seo EY, Kim WK. Effects of α-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009. 3:265–271.

23. Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q, Xu K. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010. 10:269.

24. Ho CC, Lai KC, Hsu SC, Kuo CL, Ma CY, Lin ML, Yang JS, Chung JG. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human gastric cancer AGS cells via suppressing ERK signal pathways. Hum Exp Toxicol. 2011. 30:296–306.

25. Hwang ES, Lee HJ. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp Biol Med (Maywood). 2006. 231:421–430.

27. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991. 5:2145–2154.

28. Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999. 107:11–18.

29. Murphy G, Reynolds JJ, Hembry RM. Metalloproteinases and cancer invasion and metastasis. Int J Cancer. 1989. 44:757–760.
30. Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983. 80:1337–1341.

31. Ura H, Bonfil RD, Reich R, Reddel R, Pfeifer A, Harris CC, Klein-Szanto AJ. Expression of type IV collagenase and procollagen genes and its correlation with the tumorigenic, invasive, and metastatic abilities of oncogene-transformed human bronchial epithelial cells. Cancer Res. 1989. 49:4615–4621.
32. Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991. 51:5054s–5059s.
33. Tryggvason K, Höyhtyä M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993. 24:209–218.

34. Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993. 53:5365–5369.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download