Abstract
The prevalence of atopic dermatitis (AD) in school-age children has increased in industrialized countries. As diet is one of the main factors provoking AD, some studies have suggested that food additives in processed foods could function as pseudoallergens, which comprise the non-immunoglobulin E-mediated reaction. Eosinophil cationic protein (ECP) is an eosinophil granule protein released during allergic reactions to food allergens in patients with AD. Thus, serum ECP levels may be a useful indicator of ongoing inflammatory processes in patients with AD. The purpose of this study was to investigate the effect of consuming MSG in processed foods on serum ECP levels among children with AD. This study was performed with 13 patients with AD (age, 7-11 years) who had a normal range of total IgE levels (< 300 IU/ml). All participants ate normal diets during the first week. Then, six patients were allocated to a processed food-restricted group (PRDG) and seven patients were in a general diet group (GDG). During the second week, children in the PRDG and their parents were asked to avoid eating all processed foods. On the third week, children in the PRDG were allowed all foods, as were the children in the GDG throughout the 3-week period. The subjects were asked to complete a dietary record during the trial period. Children with AD who received the dietary restriction showed decreased consumption of MSG and decreased serum ECP levels and an improved SCORing score on the atopic dermatitis index (P < 0.05). No differences in serum ECP levels or MSG consumption were observed in the GDG. Serum total IgE levels were not changed in either group. In conclusion, a reduction in MSG intake by restricting processed food consumption may lead to a decrease in serum ECP levels in children with AD and improve AD symptoms.
The prevalence of atopic dermatitis (AD) in children has increased in industrialized countries and is related to the Westernization of food habits and environmental pollution [1,2]. Various genetic, dietary, and environmental factors are potential risks provoking AD. Among these, diet is one of the main factors in patients presenting with symptoms of AD [3].
The use of convenient processed foods is common. Evidence is growing that these foods may cause adverse reactions, including immunological hypersensitivity [4]. The prevalence of adverse reactions to food additives is up to 2% in children [5] and this increases to 7% in children with AD [6]. Numerous studies have shown that foods such as cow's milk and hen's eggs directly provoke atopic symptoms in children with AD [7-9]. Although the safety of processed foods for children with AD remains controversial, current research on the safety of processed food consumed by children with AD is insufficient.
Food additives are classified by function, such as antioxidants, colorings, emulsifiers, flavorings, taste enhancers, preservatives, and stabilizers [4]. Monosodium glutamate (MSG) is a salt that forms glutamic acid, has unique flavor-enhancing qualities, and is widely used as a food additive [10]. MSG is a popular flavor enhancer and is added to many foods, particularly Asian dishes [11]. The average intake in a meal can be 3-5 g, with widely variable daily intake that may reach 12 g in some countries [12]. After Kwok reported the clinical MSG reaction called "Chinese restaurant syndrome" in 1968 [13], some studies suggested that MSG intake was positively associated with inflammatory disease [13,14]. However, other studies reported no significant adverse reactions in healthy individuals when up to 3 g MSG is given with food [15].
One recent study reported that AD is associated with food allergy [8] and that the prevalence of food allergy is 50% in patients with AD [16]. A food allergy is an immune-mediated food intolerance. The mechanism of immune-mediated adverse reactions may be divided into immunoglobulin (Ig)-E mediated or immediate-in-time and non-IgE-mediated or delayed-in-time responses [4,17]. This categorization has been extended by creating a third subgroup of mixed IgE- and non-IgE-mediated disorders such as AD, allergy asthma, and eosinophilic esophagitis [7,18]. Some studies have suggested a mechanism that associates non-IgE-mediated or delayed-in-time responses with food additives [9,19].
Eosinophils are important as effecter cells in AD allergic inflammation because they migrate from the blood into lesional skin. Eosinophils participate in the complex regulatory system that mediates inflammatory responses [20]. Eosinophil cationic protein (ECP) is one of the eosinophil granule proteins released during an allergic reaction in patients with AD. Czech et al. [21] showed that ECP had a higher correlation with total clinical score such as lichenification, loss of sleep, erythema, papules, pruritus, and excoriations than immunological parameters such as total IgE. Measuring serum ECP levels may be a useful indicator of eosinophil activity during ongoing inflammatory processes. In previous studies of adult and young patients with AD, ECP levels were elevated and significantly correlated with disease severity [22-25].
The present study investigated the effects of consuming MSG in processed foods on the serum level of ECP among school children diagnosed with AD who had a normal range of serum total IgE levels.
This study was performed with 13 patients with AD (age, 7-11 years old). All patients met the diagnostic criteria for AD as defined by Hanifin and Rajka [26]. It has been suggested that adverse reactions to food additives are non-IgE-mediated [19,20]; therefore, subjects with a normal range of serum total IgE levels (< 300 IU/ml) were selected [27].
All participants ate their normal diet during the first week. Then, six patients were allocated to a processed food-restrict group (PRDG) and seven patients to a general diet group (GDG). During the second week, children in the PRDG and their parents were asked to avoid eating all processed foods listed in Table 1 [28]. During the third week, subjects in the PRDG were allowed to eat all foods. Children in the GDG were allowed to eat all food throughout the experimental period.
All participants were asked to complete a daily dietary record during this period. Blood samples were collected on the first day of the experiment and at the end of each week for 2 or 3 weeks.
The Ethical Committee of the Seoul Allergy and Immunology Association, Seoul, Korea approved the present study, and written informed consent was obtained from each patient's parent.
Seven-day food intake dietary records were completed by all subjects. The dietary records were analyzed using the Computer Aided Nutritional Analysis Program III (Can Pro 3.0) developed by the Korean Nutrition Society. We estimated nutrient intake, frequency, and quantity of processed foods consumed for 1 week. The quantity of MSG consumed was estimated using the median value of MSG content in each food consumed, as indicated by the Korea Food and Drug Administration.
Total IgE levels were determined by an ADVIA Centaur XP Systems instrument (Siemens, Munich, Germany). Blood samples were obtained on the first day of the trial period and at the end of each week. Serum ECP levels were measured by an EIA test method using the MESACUP ECP Test (Medical & Biological Laboratories, Nagano, Japan) according to the manufacturer's guidelines.
To evaluate the clinical improvement in AD, every patient was assessed by a single observer and objectively scored for disease extent and severity using the SCORing Atopic Dermatitis (SCORAD) [29] at the end of each week for 3 weeks.
Differences in daily nutrient intake of children in the PRDG and GDG were analyzed by the t-test. Baseline characteristics, total IgE, and serum ECP levels were analyzed with nonparametric tests. The Mann-Whitney U-test was used for unpaired analyses and Wilcoxon's signed-rank test was used for paired analyses. Spearman's correlation and Spearman's partial rank-order correlation were used to determine the correlation between changes in serum ECP levels and MSG intake. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA), and P-values < 0.05 were considered significant.
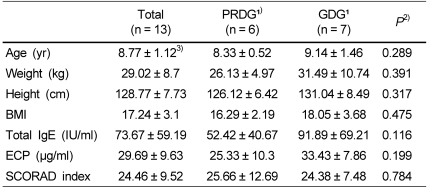
Thirteen patients (nine females and four males; mean age, 8.77 years; range, 7-11 years), who lived in Seoul, South Korea, participated in the study. Their mean body weight was 29.02 kg, and mean height was 128.77 cm. Baseline serum ECP levels of all subjects were 29.69 ± 9.63 µg/ml, and total IgE levels were 73.67 ± 59.19 IU/ml (Table 2).
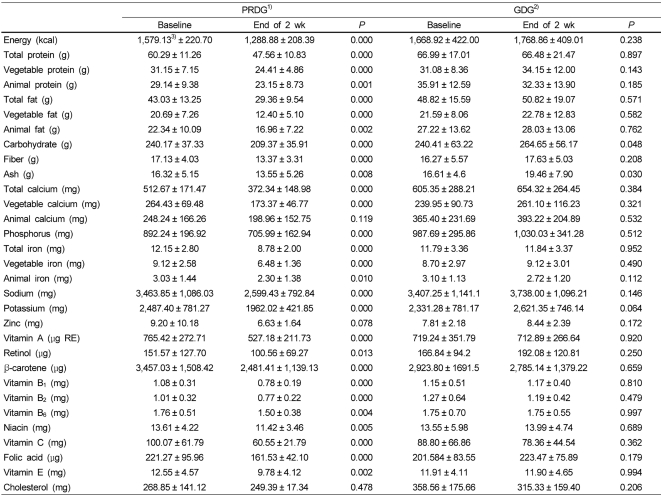
No statistically significant differences were observed between children in the PRDG and GDG for daily nutrient intake after the first week. Daily nutrient intake of children in the PRDG decreased significantly after restricting processed foods during the second week. In contrast, daily nutrient intakes of children in the GDG were similar for the entire study period (Table 3).
The estimated baseline MSG intake for children in the PRDG was 932.30 ± 230.70 mg/day. After restricting processed foods, MSG intake decreased significantly to 447.34 ± 123.83 mg/day (P = 0.028) during the second week. During the third week, children in the PRDG returned to a normal diet and their MSG intake increased to 749.37 ± 192.14 mg/day. In contrast, weekly changes in MSG intake were insignificant for children in the GDG during the trial period (Fig. 1).
Serum ECP levels in children in the PRDG decreased from 25.33 ± 10.30 to 10.25 ± 8.09 µg/ml (P = 0.028) after restricting processed foods and increased to 44.75 ± 27.42 µg/ml on the third week when the restriction was lifted (Fig. 2). Total IgE levels did not change significantly in either group throughout the experimental period (Fig. 3).
The SCORAD index in the PRDG decreased from 23.53 ± 11.68 to 12.4 ± 6.01 then increased again when the children began consuming their usual diet. Serum ECP levels and the SCORAD index were not significantly different in the GDG between the first and second week. The pattern of changes not only in serum ECP levels but also the SCORAD index was similar to that of MSG intake (Fig. 4). A significant positive correlation was observed (r = 0.629, P = 0.028) between changes in serum ECP levels and MSG intake. Furthermore, this significant correlation remained (r = 0.774, P = 0.014) after adjusting for other nutrient factors, which were significantly correlated with serum ECP levels (r = 0.650 and P = 0.022 for sodium; r = 0.727 and P = 0.007 for potassium; r = 0.587 and P = 0.044 for fiber).
Processed foods were restricted in children with AD to investigate the effect of food additives, such as MSG, on serum ECP levels. As a result, significant decreases in MSG intake occurred. Although some studies have suggested that restricting processed foods and food additives improves chronic urticaria [30,31], adverse reactions to foods and food additives have frequently been disputed as a cause for chronic urticaria [28].
After the processed food restriction, daily nutrient intakes decreased significantly in the PRDG. Decreased daily nutrient intake by restricting processed foods implied that children with AD consumed high amounts of processed foods in their normal diet and that they did not replace processed foods with non-processed foods during the dietary restriction period.
Previous research has suggested that people with allergies consume significantly higher amounts of processed foods, including high frequency intake of instant snack foods, which contain many types of food additives known to provoke atopic symptoms [32]. Among the many types of food additives, MSG is common in Korea as a flavor enhancer. Some studies have reported MSG intake is positively associated with inflammatory disease [13,14].
In the present study, ECP levels decreased after restricting processed foods containing MSG. Improvements in skin status and reduced ECP levels have been observed in patients with AD who were restricted from eating processed foods [33]. Paganelli et al. [20] measured serum ECP levels of 24 children with AD and observed higher levels of ECP compared to controls without AD. Studies have found that both the soluble interleukin-2 receptor and serum ECP levels are significantly higher in patients with AD than in those with urticaria or normal controls. A significant correlation is found between clinical severity scores in patients with AD and serum ECP levels [22-24]. Furthermore, Czech et al. [21] suggested that clinical improvement is associated with a decrease in both the clinical score and serum ECP levels.
However, serum total IgE levels were not influenced by restricting processed food in either group of our study. The type of hypersensitivity reaction that is caused by food additives might be considered a pseudoallergic reaction [33]. Pseudoallergens induce histamine, tryptase, and other pro-inflammatory mediators produced by mast cells in vivo [34] The mechanisms are only partly understood, but they are apparently based on direct activation of mast cells. The pseudoallergic reaction is not IgE-mediated and does not require prior sensitization [35]. This may explain why total IgE levels of all subjects were not affected by food additives in the present study.
We found a decrease in serum ECP levels by reducing dietary intake of MSG for 1 week. Further research should examine more closely whether high intakes of MSG provoke AD symptoms.
This study had several limitations. Double-blind placebo controlled food challenges are the gold standard for confirming a food allergy [36]. As blinding of food containing MSG is very difficult, the present study did not include a food challenge. However, we showed a decrease in serum ECP levels by restricting processed food intake for 1 week among children with AD that increased to former levels when participants returned to their normal diets.
Further studies are necessary to provide clear evidence of an association between MSG intake and increasing or decreasing serum ECP level or other cytokines.
In conclusion, serum ECP levels decreased following decreased intake of processed food, suggesting an improvement in inflammatory processes in children with AD. MSG influenced ECP levels but not IgE levels, suggesting that MSG may be involved non-IgE-mediated food allergies.
References
1. Beasley R. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998; 351:1225–1232. PMID: 9643741.

2. von Mutius E. The environmental predictors of allergic disease. J Allergy Clin Immunol. 2000; 105:9–19. PMID: 10629447.

3. Yoon SP, Kim BS, Lee JH, Lee SC, Kim YK. The environment and lifestyles of atopic dermatitis patients. Korean J Dermatol. 1999; 37:983–991.
4. Wilson BG, Bahna SL. Adverse reactions to food additives. Ann Allergy Asthma Immunol. 2005; 95:499–507. PMID: 16400887.

5. Fuglsang G, Madsen C, Saval P, Osterballe O. Prevalence of intolerance to food additives among Danish school children. Pediatr Allergy Immunol. 1993; 4:123–129. PMID: 8220800.

6. Fuglsang G, Madsen G, Halken S, Jørgensen S, Ostergaard PA, Osterballe O. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994; 49:31–37. PMID: 8198237.

7. Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005; 128:1089–1113. PMID: 15825090.

8. Rancé F. Food allergy in children suffering from atopic eczema. Pediatr Allergy Immunol. 2008; 19:279–284. PMID: 18397414.

10. Williams AN, Woessner KM. Monosodium glutamate 'allergy': menace or myth? Clin Exp Allergy. 2009; 39:640–646. PMID: 19389112.

11. Randhawa S, Bahna SL. Hypersensitivity reactions to food additives. Curr Opin Allergy Clin Immunol. 2009; 9:278–283. PMID: 19390435.

12. Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R. Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr. 2007; 61:304–313. PMID: 16957679.

14. Raiten DJ, Talbot JM, Fisher KD. Executive summary from the report: analysis of adverse reactions to monosodium glutamate (MSG). J Nutr. 1995; 125:2891S–2906S. PMID: 7472671.
15. Zanda G, Franciosi P, Tognoni G, Rizzo M, Standen SM, Morselli PL, Garattini S. A double blind study on the effects of monosodium glutamate in man. Biomedicine. 1973; 19:202–204.
16. Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985; 107:669–675. PMID: 4056964.

17. Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, Kowalski ML, Mygind N, Ring J, van Cauwenberge P, van Hage-Hamsten M, Wüthrich B. EAACI (the European Academy of Allergology and Cinical Immunology) nomenclature task force. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001; 56:813–824. PMID: 11551246.

18. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004; 113:832–836. PMID: 15131563.

19. Warrington RJ, Sauder PJ, McPhillips S. Cell-mediated immune responses to artificial food additives in chronic urticaria. Clin Allergy. 1986; 16:527–533. PMID: 3539408.

20. Wardlaw AJ. Eosinophils in the 1990s: new perspectives on their role in health and disease. Postgrad Med J. 1994; 70:536–552. PMID: 7937446.

21. Czech W, Krutmann J, Schöpf E, Kapp A. Serum eosinophil cationic protein (ECP) is a sensitive measure for disease activity in atopic dermatitis. Br J Dermatol. 1992; 126:351–355. PMID: 1571256.

22. Furue M, Sugiyama H, Tsukamoto K, Ohtake N, Tamaki K. Serum soluble IL-2 receptor (sIL-2R) and eosinophil cationic protein (ECP) levels in atopic dermatitis. J Dermatol Sci. 1994; 7:89–95. PMID: 8060919.

23. Halmerbauer G, Frischer T, Koller DY. Monitoring of disease activity by measurement of inflammatory markers in atopic dermatitis in childhood. Allergy. 1997; 52:765–769. PMID: 9265994.

24. Kägi MK, Joller-Jemelka H, Wüthrich B. Correlation of eosinophils, eosinophil cationic protein and soluble interleukin-2 receptor with the clinical activity of atopic dermatitis. Dermatology. 1992; 185:88–92. PMID: 1421636.
25. Paganelli R, Fanales-Belasio E, Carmini D, Scala E, Meglio P, Businco L, Aiuti F. Serum eosinophil cationic protein in patients with atopic dermatitis. Int Arch Allergy Appl Immunol. 1991; 96:175–178. PMID: 1769747.

26. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
27. Jackola DR, Pierson-Mullany LK, Daniels LR, Corazalla E, Rosenberg A, Blumenthal MN. Robustness into advanced age of atopy-specific mechanisms in atopy-prone families. J Gerontol A Biol Sci Med Sci. 2003; 58:99–107. PMID: 12586846.

28. Zuberbier T, Chantraine-Hess S, Hartmann K, Czarnetzki BM. Pseudoallergen-free diet in the treatment of chronic urticaria. A prospective study. Acta Derm Venereol. 1995; 75:484–487. PMID: 8651031.
29. Stalder JF, Taieb A, Atherton DJ, Bieber T, Bonifazi E, Broberg A, Calza A, Coleman R, De Prost Y, Diepgen TL, Gelmetti C, Giannetti A, Harper J, Kunz B, Lachapelle JM, Langeland T, Lever R, Oranje AP, Queille-Roussel C. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31. PMID: 8435513.
30. Juhlin L. Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol. 1981; 104:369–381. PMID: 7236502.

31. Magerl M, Pisarevskaja D, Scheufele R, Zuberbier T, Maurer M. Effects of a pseudoallergen-free diet on chronic spontaneous urticaria: a prospective trial. Allergy. 2010; 65:78–83. PMID: 19796222.

32. Yang SH, Kim EJ, Kim YN, Seong KS, Kim SS, Han CK, Lee BH. Comparison of eating habits and dietary intake patterns between people with and without Allergy. Korean J Nutr. 2009; 42:523–535.

33. Worm M, Ehlers I, Sterry W, Zuberbier T. Clinical relevance of food additives in adult patients with atopic dermatitis. Clin Exp Allergy. 2000; 30:407–414. PMID: 10691900.

34. Murdoch RD, Pollock I, Young E, Lessof MH. Food additive-induced urticaria: studies of mediator release during provocation tests. J R Coll Physicians Lond. 1987; 21:262–266. PMID: 2445986.
35. Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997; 136:871–877. PMID: 9217819.

36. Burks AW, Sampson HA. Diagnostic approaches to the patient with suspected food allergies. J Pediatr. 1992; 121:S64–S71. PMID: 1280298.

Fig. 1
Changes in MSG intake by restricting processed foods. PRDG, processed foods restricted diet group (n = 6); GDG, general diet group (n = 7). The Mann-Whitney U-test was used for unpaired analyses, and the Wilcoxon signed-rank test was used for paired analyses. A P-value of < 0.05 was considered significant.
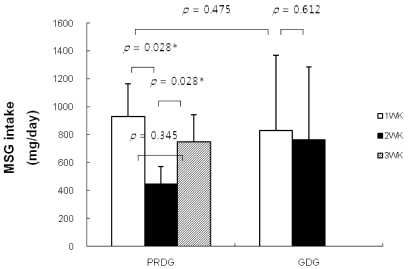
Fig. 2
Changes in serum eosinophil cationic protein (ECP) levels by restricting processed foods. PRDG, processed foods restricted diet group (n = 6); GDG, general diet group (n = 7). The Mann-Whitney U-test was used for unpaired analyses, and the Wilcoxon signed-rank test was used for paired analyses. A P-value of < 0.05 was considered significant.
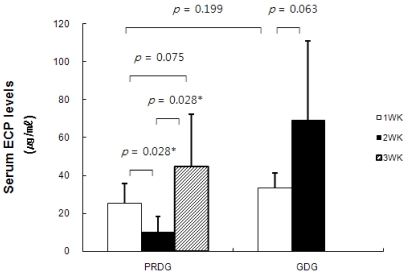
Fig. 3
Changes in serum total immunoglobulin-E (IgE) levels by restricting processed foods. PRDG, processed foods restricted diet group (n = 6); GDG, general diet group (n = 7). The Mann-Whitney U-test was used for unpaired analyses, and the Wilcoxon signed-rank test was used for paired analyses. A P-value of < 0.05 was considered significant.
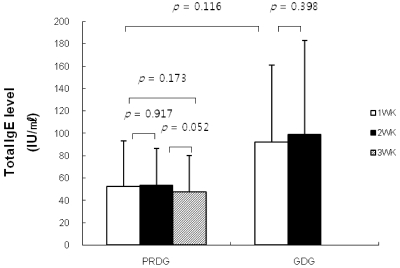
Fig. 4
Changes in the SCORing Atopic Dermatitis (SCORAD) index by restricting processed foods. PRDG, processed foods restricted diet group (n = 6); GDG, general diet group (n = 7). The Mann-Whitney U-test was used for unpaired analyses, and the Wilcoxon signed-rank test was used for paired analyses. A P-value of < 0.05 was considered significant.
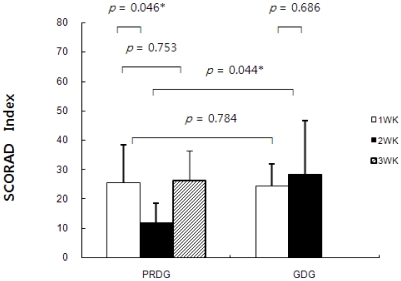
Table 1
Forbidden foods in the processed food-restricted group: All foods containing food additives and industrially processed food
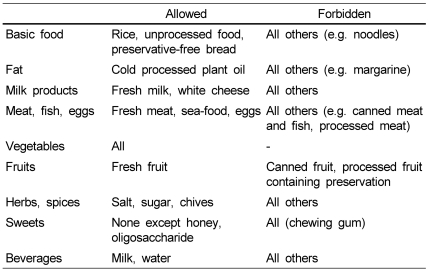
This table is based on Zuberbier et al. [28].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download