Abstract
Diets based on carbohydrates increase rapidly the blood glucose level due to the fast conversion of carbohydrates to glucose. High glucose diets have been known to induce many lifestyle diseases. Here, we demonstrated that high glucose diet shortened the lifespan of Caenorhabditis elegans through apoptosis induction. Control adult groups without glucose diet lived for 30 days, whereas animals fed 10 mg/L of D-glucose lived only for 20 days. The reduction of lifespan by glucose diet showed a dose-dependent profile in the concentration range of glucose from 1 to 20 mg/L. Aging effect of high glucose diet was examined by measurement of response time for locomotion after stimulating movement of the animals by touching. Glucose diet decreased the locomotion capacity of the animals during mid-adulthood. High glucose diets also induced ectopic apoptosis in the body of C. elegans, which is a potent mechanism that can explain the shortened lifespan and aging. Apoptotic cell corpses stained with SYTO 12 were found in the worms fed 10 mg/L of glucose. Mutation of core apoptotic regulatory genes, CED-3 and CED-4, inhibited the reduction of viability induced by high glucose diet, which indicates that these regulators were required for glucose-induced apoptosis or lifespan shortening. Thus, we conclude that high glucose diets have potential for inducing ectopic apoptosis in the body, resulting in a shortened lifespan accompanied with loss of locomotion capacity.
In most industrialized cities and Asian countries, dietary habits tend to be characterized by higher glycemic index (GI) [1]. Since GI is a measure of the effect of carbohydrates on the blood sugar level, a diet with high content of carbohydrates results in higher GI. A lower GI, however, indicates slow rates of carbohydrate digestion with lower insulin demand. High GI diets are known to be related to diseases in adults such as obesity, heart diseases, and diabetes [2-4]. However, the biochemical or genetic mechanisms behind high GI diets have not yet been illuminated clearly. Some of the possible mechanisms driving the onset of such diseases induced by high GI diets have been linked to apoptotic cell death and alteration of the insulin signaling pathway [5,6]. A working model to explain the interaction between high GI diets and the insulin signaling pathway has been developed; however, the role of apoptosis leading to a short lifespan has not been illuminated yet.
Apoptosis is the onset of programmed cell death during the development of multicellular organisms for the removal of unnecessary cells or tissues [7]. Since alterations of cellular apoptosis are relevant to a number of human diseases, genetic or biochemical approaches to control apoptotic cell death have been investigated in many in vitro and in vivo model organisms [8,9]. The genetic mechanism of apoptosis was first observed in the model organism nematode Caenorhabditis elegans [10]. Once CED-9, a mammalian Bcl-2-like protein, is inactivated by intrinsic or extrinsic factors, CED-4, an Apaf-1-like protein, can be activated, thus initiating the caspase protein CED-3, which eventually executes apoptosis in C. elegans [11-13].
Most nutrition research has been investigated using mammals, including mouse and human or in vitro tissue cultures. Here, we suggest that nematode C. elegans may be an ideal model organism to study nutrition in terms of genetics, development, and behavior. In comparison with mice, these animals have a short lifecycle (30 days) and large brood size (300 eggs) [14]. By feeding C. elegans with bacterial strains expressing siRNA, genetic mutant animals can be generated in just 1 day [15].
In this study, we examined the effects of high glucose diets on the lifespan of a model animal, C. elegans. The mechanism explaining the shortened lifespan of the animal was elucidated by showing evidence of apoptosis induced by high glucose intake.
Strains used in this study include: N2 [wild type], MT1522 [ced-3(n717) IV], MT3002 [ced-3(n1286) IV], MT2547 [ced-4 (n1162) III], and MT5287 [ced-4(n1894) III].
Strains were cultured and maintained as previously described [13]. Synchronization of worms was achieved by preparing eggs from gravid adults using a solution containing 1.5% NaOCl and 1.5 M NaOH. Eggs were washed with M9 buffer and then allowed to hatch overnight on NGM (normal growth media) agar plates without bacteria.
Lifespan analysis was carried out at 20℃ on day 1 adult animals by placing larvae worms at L4 stage 1 day before starting lifespan analysis [16]. Adult animals were transferred to fresh NGM plates using a platinum pick wire every day to prevent adults from generating newborn progenies. The survival ratio was calculated from the percentage of living worms out of the total number of worms, including living and dead animals. For the high glucose diets, 1-20 mg/L of D-Glucose was added to the NGM agar plates.
C. elegans animals were transferred to 5% agar pads on a plain glass slide and then anesthetized by adding 5 mM levamisole solution onto the pad. Apoptotic cell corpses were analyzed under a Zeiss microscope with 63 × and 100 × DIC objectives.
To identify apoptotic cells induced by high glucose diet, animals were stained with SYTO 12 (Molecular Probes) as previously described [17]. The adult worms were incubated in 50 µM SYTO 12 for 4 hrs at room temperature and then seeded on bacterial lawns to reduce the amount of stained bacteria in the gut. After 30 min, animals were washed with M9 buffer, mounted on the agar pad slide glass, and observed under a Zeiss microscope equipped with a red fluorescence filter.
The locomotion capacity of old animals was evaluated by measuring the response time to touching of the animal head. The adult worms at day 10 were placed on the empty agar plate without bacterial food, after which their heads were touched using an eyelash under a stereo microscope. Motion pictures of the worms were recorded by a camera connected to the microscope. Time of locomotion was measured from the recorded motion pictures to determine the motion capacity of adult worms when stimulated by touching.
In general, wild-type adult Caenorhabditis elegans animals are known to live for 30 days. In a previous study, diets of 0.1~2% (w/v) glucose resulted in the reduction of lifespan in C. elegans [18]. In order to examine the effects of glucose diet on the lifespan of these animals, 10 mg/L of D-glucose was added to their foods. C. elegans that were fed glucose lived for only 20 days (Fig. 1A). At 10 days of adulthood, about 90% of the control animals were still alive, whereas only around 60% of the worms fed glucose survived (P < 0.01, student t-test).
In the concentration range of glucose from 1 to 20 mg/L, the percentage of animal survival was reduced in a dose-dependent manner (Fig. 1B). When animals were fed 1 mg/L of D-glucose, the profile of animal survival was similar to that of control with a survival ratio of 88.5%, which is 23% higher than worms fed 10 mg/L of glucose. However, the number of dead adult worms increased by day 10 as the concentration of glucose increased. The survival ratio was significantly reduced by 49.5% in the animal population fed 20 mg/L of glucose (P < 0.01, student t-test).
The effect of high glucose diet on motion ability of the animals was investigated by measuring the time of response locomotion when the worms were touched. In general, C. elegans moved backward quickly when its head was touched or hit by something. When the animals lost their active locomotion capacity, they failed to move or stopped moving quickly despite touch stimulation. The locomotion time of control adult worms at day 10 was 2.57 ± 0.35 sec, which was used to normalize the response time of animals fed high glucose diets (Fig. 2). Although 1 mg/L of glucose diet did not affect the survival ratio of C. elegans (Fig. 1A), this smaller concentration of glucose reduced the locomotion capacity of the worms (82.5% less than control, P < 0.01). As the level of glucose concentration increased, slower locomotion was observed in adults at day 10: 59.5% at 10 mg/L and 47.5% at 20 mg/L. These results imply that diets based on high glucose level induce aging in animals.
A possible mechanism explaining the relevance of high glucose diet in apoptosis was reported using an in vitro system [19]. When cells forming the body of C. elegans undergo apoptosis, cell corpses with a circular button shape are detected in the organism. Since chromatin in the nucleus becomes condensed in apoptotic cells, cells emitting fluorescence are observed when the animals are stained with DNA-intercalating fluorescent dyes such as SYTO 12 and Acridine Orange. In the animals fed 10 mg/L of glucose, cell corpses stained with SYTO 12 were detected at day 10 of adulthood (Fig. 3). The average number of cell corpses per worm fed 10 mg/L of glucose was 2.42 ± 1.68 at 10 days in adulthood.
Core regulatory genes required for the apoptotic pathway of C. elegans have been identified in the past decade [10-14]. CED-3 and CED-4 proteins are core genetic regulators that execute the apoptotic process. Using worm strains with mutated ced-3 or ced-4 genes, we investigated whether or not loss-of-function mutations in pro-apoptotic genes can rescue high glucose-induced ectopic apoptosis, resulting in increased survival. As shown in Fig. 4, mutation of pro-apoptotic ced-3 and ced-4 reduced lifespan shortening induced by high glucose diet. Around 10% of the worms of each mutant type fed high glucose diet had died by 10 day of adulthood, whereas only about 30% of the wild-type N2 worms died.
Little is known about how dietary habits and nutrition balance affect the determination of lifespan. It has been reported in many in vivo models that dietary restriction increases the lifespan of animals [20,21]; however, disorganized restriction of diets tends to collapse nutrition balance and becomes a large hindrance to physical and social activities. A major finding of this study was that high glucose diet has the potential for shortening lifespan of animals by inducing ectopic apoptosis in the body. Since carbohydrates are easily converted to glucose by digestion systems, diets based on foods composed of mainly carbohydrates are potent inducers of ectopic apoptosis, resulting in decrease of lifespan.
Since apoptosis in C. elegans is known to be very conservative [13], we were surprised to find ectopic cell corpses in the adult animals. Most apoptotic events occur during the embryonic developmental stage of C. elegans, and the activation and expression levels of pro-apoptotic proteins such as CED-3 and CED-4 are relatively lower in the adult stage than embryonic or larval stage [12]. Given that toxic chemical agents reduce the viability of C. elegans in their early adulthood, glucose, which starts to reduce lifespan during mid or late adulthood, thus acts like an aging-promoting agent rather than toxic drug. The loss of locomotion capacity shown in our results also supports the pro-aging effects of glucose diets.
In an in vitro system, the addition of high glucose induces apoptosis in human umbilical vein endothelial cells [19]. It was also found that glucose diet reduces the lifespan of C. elegans through repressing the activity of transcription factors involved in extending lifespan [18]. Such transcription factors are inactivated by insulin signaling executed by DAF-16 and HSF-1 [22,23]. The level of insulin in the blood increases quickly after intake of high glucose foods and then decreases soon thereafter, making insulin a potent mediator of the effects of high glucose diets on aging in humans. Such fluctuation of the blood insulin level has the potential to induce strong insulin resistance, resulting in type 2 diabetes [4]. Since high glucose diets increase the total energy content in C. elegans, it is necessary to investigate whether or not higher energy content upon glucose consumption results in a shortened lifespan in adult animals.
The data from this study suggest that the lifespan of animals may be shortened by high glucose diets. Although a low level of glucose did not decrease lifespan, an aging effect in locomotion capacity was observed during mid-adulthood. Decreases in lifespan and locomotion ability were dependent on glucose concentration in the foods. Ectopic apoptosis was induced by high glucose diets, which led to the reduction of the survival ratio. Loss-of-function mutation in pro-apoptotic genes rescued these lifespan-shortening effects. Those results demonstrate that apoptosis induction by high glucose diets results in shortened lifespan in C. elegans.
Figures and Tables
Fig. 1
High glucose diets shorten lifespan of C. elegans. (A) Effect of glucose diet (10 mg/L) on lifespan of animals. In each experiment, 100 animals at L4 larvae stage were initially placed on NGM agar plates and transferred to new fresh plates every day. (B) Viability of animals fed various concentrations of glucose at day 10 during adulthood. Values represent means ± SD of quadruple measurements (n = 400, *P < 0.01).
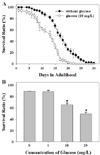
Fig. 2
Glucose diets decrease locomotion capacity of adult C. elegans. Heads of animals were touched by an eyelash, and movement was recorded by a camera connected to a microscope until movement stopped. From the motion pictures, the time of locomotion was measured. Values represent means ± SD of 100 animals fed each concentration of glucose (*P < 0.01).

Fig. 3
Glucose diets induce ectopic apoptosis in C. elegans. (A) DIC image of animal with apoptotic cell corpses (arrow). (B) Fluorescence image of animal with glowing cell corpses stained by SYTO 12 (arrow). Images were taken under a Zeiss microscope using × 100 objective lens with DIC or red fluorescence filters. Scale bars are 20 µm.
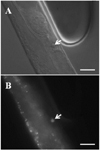
Fig. 4
CED-3 and CED-4 are required for glucose diet-induced apoptosis. Viabilities increased in the strains with mutations of pro-apoptotic regulators, CED-3 and CED-4, at day 10 of adulthood in comparison with wild-type N2 worms fed 10 mg/L of glucose. Values represent means ± SD of quadruple measurements (n = 400, *P < 0.01).

References
1. Lin MH, Wu MC, Lu S, Lin J. Glycemic index, glycemic load and insulinemic index of Chinese starchy foods. World J Gastroenterol. 2010. 16:4973–4979.

2. Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002. 76:281S–285S.

3. Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010. 55:1283–1299.
4. Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr. 2010. 104:465–473.

5. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001. 131:3109S–3120S.

6. Minich DM, Bland JS. Dietary management of the metabolic syndrome beyond macronutrients. Nutr Rev. 2008. 66:429–444.

7. Choi SS, Rhee WJ, Park TH. Inhibition of human cell apoptosis by silkworm hemolymph. Biotechnol Prog. 2002. 18:874–878.

8. Choi SS, Rhee WJ, Park TH. Beneficial effect of silkworm hemolymph on a CHO cell system: Inhibition of apoptosis and increase of EPO production. Biotechnol Bioeng. 2005. 91:793–800.

9. Choi SS, Rhee WJ, Kim EJ, Park TH. Enhancement of recombinant protein production in Chinese hamster ovary cells through anti-apoptosis engineering using 30Kc6 gene. Biotechnol Bioeng. 2006. 95:459–467.

10. Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986. 44:817–829.

11. Yuan JY, Horvitz HR. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990. 138:33–41.

12. Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992. 356:494–499.

13. Hengartner MO, Horvitz HR. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature. 1994. 369:318–320.

14. Potts MB, Cameron S. Cell lineage and cell death: Caenorhabditis elegans and cancer research. Nat Rev Cancer. 2011. 11:50–58.

15. Lamitina T. Functional genomic approaches in C. elegans. Methods Mol Biol. 2006. 351:127–138.
16. Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003. 300:1142–1145.

17. Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999. 126:1011–1022.

18. Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009. 10:379–391.

19. Zhao H, Liu G, Wang Q, Ding L, Cai H, Jiang H, Xin Z. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochem Biophys Res Commun. 2007. 362:677–681.

20. Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011. 10:5–9.

21. Hoffman DJ. Early nutrition and adult health: perspectives for international and community nutrition programs and policies. Nutr Res Pract. 2010. 4:449–454.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download