Abstract
We conducted this study to examine the effects of safflower seed granular tea containing physiologically active polyphenols on antioxidative activities and bone metabolism. Forty postmenopausal women ages 49 to 64-years were recruited from Daegu and Gyeongbuk and were randomly assigned to either a safflower tea supplement (Saf-tea) group (n = 27) or a placebo group (n = 13). The Saf-tea group received 20 g of safflower seed granule tea per day containing a 13% ethanol extract of defatted safflower seeds, whereas the placebo group received a similar type of tea that lacked the ethanol extract. No significant changes in nutrient intake for either the placebo or Saf-tea groups were observed before or after the study period, except vitamin A intake increased after 6 months in the Saf-tea group. Dietary phytoestrogen intakes were similar in the Saf-tea group (60.3 mg) and placebo group (52.5 mg). Significant increases in plasma genistein and enterolactone were observed in the Saf-tea group. After 6 months of supplementation, serum levels of antioxidant vitamins such as α-tocopherol and ascorbic acid increased significantly, and TBARS levels decreased in the Saf-tea group compared to the placebo group. Serum osteocalcin levels were reduced (P < 0.05) in the Saf-tea group after 6 months, whereas serum osteocalcin did not change in the placebo group. Urinary deoxypyridinoline/creatinine excretion was not different between the two groups at baseline, and did not change in either group after 6 months. Bone mineral density decreased significantly in the placebo group (P < 0.01) but not in the supplemented group. It was concluded that polyphenols (72 mg/day), including serotonin derivatives, in the Saf-tea had both antioxidant and potential bone protecting effects in postmenopausal women without liver toxicity.
Safflower (Cathamus tinctorius L.) seeds are a good source of edible oil rich in α-linoleic acid (n-6), which has a hypocholesterolemic effect. Safflower seeds have long been used clinically in Korea to promote bone formation and to prevent osteoporosis [1]. Several reports suggest that safflower seed powder or extract promotes recovery from bone fractures and stimulates differentiation of osteoblasts in rats [2-5]. Our previous studies showed that mixed polyphenol compounds extracted from defatted safflower seeds stimulates proliferation of ROS 17/2.8 osteoblast-like cells [6,7]. Furthermore, feeding the seed powder [6] and its defatted ethanol extract [7] markedly attenuates bone loss in ovariectomized rats. Kang et al. [8] and Kim et al. [9] isolated and identified phenolic compounds from roasted safflower seeds, and found that safflower seeds were a rich source of not only phytoestrogen-like flavones (acacetin and acacetin 7-O-glucoside) and lignans (matairesinol and 8-hydroxyarctigenin), but also serotonin derivatives (N-feruloylserotonin and N-(p-coumaroyl) serotonin) that exhibit antioxidant activity in vitro [8,9]. More recently, we showed that a water extract of germinated safflower seeds has proliferative and differentiation effects on mouse calvarial bone cells, and a major component for the effects was trachelogenin [10].
Therefore, seed components with bone-forming effects can be utilized as health foods to prevent osteoporosis in postmenopausal women. Safflower seed tablets have been formulated as a health food by simply grinding and pressing seed powder, but consumers complained due to the rancidity of the tablet contents during storage. To make up for the shortcomings of such tablets, tea-bag [11] and drink-type products [11-13] have been prepared using safflower seed powder and ethanol extracts. These products have been tested for antioxidant activity [12], sensory qualities, and product quality characteristics [11-13] but not for their physiological effects in animals and humans. We prepared a granule-type tea, including an ethanol extract of defatted safflower seeds, and reported the hypolipidemic and of the safflower and placebo teas antioxidant effects of the tea in ovariectomized rats [14]. In the present study, we supplemented with the same safflower granular tea to determine the effects on bone metabolism and antioxidant status markers in postmenopausal women.
Subjects living in the Daegu-Gyeongbuk area were selected, including 50 post-menopausal women with ages between 49 and 64-years who had not been treated with hormone replacement therapy or any other medication affecting hormone status. Age, lifestyle factors, and gynecological and obstetric history of the subjects were investigated through a general survey questionnaire. This study was approved by the ethics committee of the Catholic University of Daegu, and informed consent was obtained from each subject. The 50 subjects were randomly assigned to a safflower tea (Saf-tea) group of 34 subjects and a placebo group of 16 subjects. Of the 50 subjects, seven from the Saf-tea group and three from the placebo group withdrew from the study during the 6-month supplementation period, so a total of 40 subjects completed the study. The number of subjects in the placebo group was rather small compared with that of the test group, because it was difficult to recruit more subjects for the placebo group due to ethical reasons.
Five hundred grams of safflower seeds (safflower seeds: Carthamus tinctorious L.) harvested from a farm in Uisong, Geyongbuk, Korea were washed, roasted at 200℃ for 5 min, ground, and extracted twice with hexane to remove lipids under reflux. The defatted seeds were further extracted with 80% ethanol, and the extract was used to prepare the granular safflower tea. The safflower tea contained 13% extract (w/w), ssangwha tea extract, soy isoflavones, glucose, mannitol, maltodextrin, lactose, and sodium silicoalumenate (Table 1). The ssangwha tea extract was added to help blood circulation as well as reduce the bitter taste of the safflower seed extract. A small amount of soy isoflavones was added to enhance the isoflavone effect in the tea but it was not expected to have any effect alone. Glucose was added for palatability, and the remaining ingredients were needed to formulate the tea granules. Ten grams of tea granules were packaged in each pack, and two packs were taken daily by the subjects in the morning and evening, respectively. Safflower polyphenol content was estimated to be 72 mg including 18 mg of serotonin derivatives, which was comparable to the amount recommended for daily intake of safflower tablets that had been for sale. The placebo tea was prepared the same as the safflower tea except that the safflower extract and soy isoflavones were replaced with maltodextrin.
The subjects who consented to participate were individually interviewed to obtain information on their general and health-related characteristics, including diet and anthropometric indices. The subjects were also advised not to change their usual diet and health-related habits during the test period. One week later, fasting blood samples were taken, and bone density was measured for baseline data. Each subject was then given enough Saf-tea or placebo to last 1 month in a double-blind fashion. They were instructed to take two packs in warm water per day, i.e., one packet each in the morning and the evening. The same procedure was repeated until completion of the 6-month study. Compliance for taking the Saf-tea or placebo was encouraged by phone calls in addition to counseling as needed for any complications that the subjects encountered. During the 6-month test period, blood samples were taken at months 3 and 6, and life style factors, anthropometric indices, dietary nutrient intakes, and bone density were examined at month 6 by the same procedure as used for baseline measurements.
The questionnaire included questions about demographic characteristics and lifestyle and maternal factors. The selected lifestyle and maternal factors were cigarette smoking, alcohol intake and physical exercise, type of menopause, and experience with contraceptive pills or hormone replacement. Dietary intake data were collected by a trained dietitian using the 24-hr recall method at months 0 and 6 and were analyzed for energy and nutrient contents using the computer aided nutritional analysis, version 3.0 program developed by The Korean Nutrition Society (2005). Phytoestrogen intake from the diet was estimated by food frequency, as described in a previous study [15] and that from safflower tea was estimated by the safflower seed polyphenol contents reported by Kang et al. [8].
Serum genistein was quantified using time-resolved fluoroimmunoassay with a Labmaster (Turku, Finland) Genestein kit (1212-2003), and enterolactone was quantified in the same manner with a Labmaster Enterolactone kit (1212-2001). Serum contents of α-tocopherol and retinol were determined by high performance liquid chromatography [16] with slight modifications, after serum samples were extracted with hexane. A C-18 µBondapak (300 × 8 mm) stainless steel column (Waters, Milford, MA, USA) was used, and the analysis was performed isocratically with methanol/H2O (97:3,v/v) as the mobile phase and ultraviolet detection at 292 nm. Serum thiobarbituric acid reactive substances (TBARS) were measured by the methods of Yagi [17] using 1,1,3,3,-tetraethoxypropane as the standard. Serum osteocalcin was measured by the IRMA method with a Osteocalcin-IRMA kit from Biosourse™ (Invitrogen, Carlsbad, CA, USA). Fasting blood samples were analyzed for serum alanine amintransferase (ALT), aspartate amintransferase (AST), blood urea nitrogen (BUN), and creatinine with an automated analyzer (Cobas Integra 700, Roche, Switzerland).
Bone mineral density (BMD) of the lumbar spine (L1-L4) was measured using dual energy X-ray absorptiometry (Hologic QDR-4500, Waltham, MA, U.S.A) at baseline and after 6 months of tea supplementation. Regions of interest for the lumbar spine were defined according to the manufacturer's guidelines. All measurements were made and analyzed by experienced operators at Catholic University's Daegu Hospital. The in vivo precision error for BMD was 1%.
A chi-square-test was used to investigate if there were any differences in general characteristics and lifestyle factors between the test and placebo groups at baseline. Paired t-tests were used to compare the blood analysis, anthropometric and dietary measurement, and BMD data in the test and placebo groups before and after supplementation. An analysis of covariance (ANCOVA) was used to evaluate differences in values before and after supplementation between the test and placebo groups. All analyses were performed using the SPSS package (SPSS, Inc., Chicago, IL, USA).
The ages of the subjects ranged from 49 to 64 years, with a mean ± standard deviation of 54.0 ± 1.9 years for the placebo group and 55.6 ± 3.6 years for the Saf-tea group. Both groups had similar heights, weights, body mass indices (BMI), and waist-hip ratios (WHR), which did not change in the placebo or Saf-tea groups during the 6-month supplementation period, except for a slight increase in WHR in the Saf-tea group (Table 2). The maternal and menopausal characteristics of the subjects were not different between the placebo and Saf-tea groups, and menopause was confirmed in both groups by estradiol levels lower than 30-400 pg/mL. Drinking, smoking, and exercise habits were similar between the placebo and the Saf-tea groups and did not change during the 6-month supplementation period.
Table 3 presents the dietary intake of nutrients, calcium, and vitamins A, C, and E in the placebo and Saf-tea groups before and after 6 months of supplementation. The Saf-tea group appeared to have a slightly higher energy intake than the placebo group at baseline and this seemed to be due to a somewhat higher intake of carbohydrate. But throughout the study period, no significant changes in either the placebo or Saf-tea group were observed before and after supplementation, except for vitamin A intake, which was higher at 6 months than at baseline in the Saf-tea group. After 6 months, changes in nutrient intake, including other nutrients not shown in Table 3, did not differ between the placebo and Saf-tea groups.
Daily phytoestrogen intake by the subjects was estimated from the diet at baseline and from the safflower tea (Table 4). The dietary phytoestrogens included isoflavones (daidzein, genistein, formononectin, and biochanin A), coumesterol, and ligans (matairesinol, secoisolarciresinol, enterolactone, and enteroldiol). Daidzein and genistein comprised the majority of dietary phytoestrogen isoflavones at 70-75%, whereas coumestetol and lignan were found at 22-28% and 2-4%, respectively. The intake of total dietary phytoestrogens appeared higher, although not significantly, in the Saf-tea group that was supplemented with an additional 54.2 mg of phytoestrogen plus 18 mg of serotonin derivatives from the tea. This resulted in a more than two-fold higher intake of phytoestrogen and serotonin derivatives in the Saf-tea group than the placebo group.
Several kinds of phytoestrogens were ingested by the subjects, but only serum levels of genestein and enterolactone, which are major metabolites of isoflavones and lignans, were measured in this study. The concentrations of serum genistein in the placebo and Saf-tea groups were 192 ± 153 and 178 ± 126 nmol/L, respectively, at baseline, which were not significantly different (Table 5). However, serum genistein levels increased significantly in the Saf-tea group following 6 months of tea supplementation compared to baseline values, and differences in the genestein levels of the placebo group before and after supplementation were observed. The same result was found for changes in serum enterolactone levels. The enterolactone level at baseline (37 ± 24 nmol/L) in the Saf-tea group almost doubled to 68 ± 42 nmol/L after Saf-tea supplementation, whereas it decreased slightly in the placebo group.
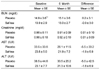
Table 6 shows serum osteocalcin levels, urinary deoxypyridinoline (DPD) excretion, and lumbar-spine BMD at several bone locations in the placebo and Saf-tea study groups before and after 6 months of tea supplementation. Serum osteocalcin did not change in the placebo group but decreased in the Saf-tea group after 6 months; however, the Saf-tea group did not show greater decreases than the placebo group for the differences between the baseline and 6-month values (P = 0.076 by ANCOVA). Urinary DPD excretion was not changed by Saf-tea supplementation. BMD (L1-L4) decreased in the placebo group but not in the Saf-tea group after 6 months of supplementation, although the differences between the baseline and 6-month values of the two groups did not show safflower tea effects.
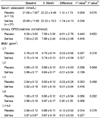
Fig. 1 shows the serum levels of retinol, α-tocopherol, and ascorbic acid for the study subjects before and during 6 months of supplementation with the placebo and Saf-tea. Levels of each vitamin were not different in the placebo and Saf-tea groups at baseline. Retinol levels did not change after 3 months but increased both in the placebo (P = 0.011) and Saf-tea tea (P = 0.000) groups after 6 months (both 0.46 ± 0.10 µg/ml) compared to baseline values (0.53 ± 0.09 and 0.58 ± 0.14 µg/ml, respectively), and no difference (P = 0.168 by ANCOVA) in retinol level were observed between the placebo and Saf-tea groups after 6 months of supplementation (Fig. 1A). α-Tocopherol appeared to decrease in the placebo group after 3 months but returned to baseline after 6 months (Fig. 1B). However, α-tocopherol increased significantly in the Saf-tea group (P = 0.000) after 6 months (12.75 ± 4.36 µg/ml), compared to the value (9.00 ± 2.26 µg/ml) at baseline and at 3 months. Differences between baseline and 6-month α-tocopherol values were significantly higher in the Saf-tea group than in the placebo group (P = 0.029 by ANCOVA). Vitamin C levels appeared to be the same in the placebo group but increased significantly in the Saf-tea group during the 6 months (1.10 ± 0.45 µg/ml) compared to baseline levels (0.92 ± 0.44 µg/ml, P = 0.036). Nevertheless, no difference (P = 0.175 by ANCOVA) in ascorbic acid levels was observed in the placebo and Saf-tea groups after 6 months of supplementation (Fig. 1C). Fig. 2 shows serum TBARS levels in the study groups during tea supplementation. TBARS levels did not change in the placebo group but appeared to continue to decrease in the Saf-tea group during the 6 months, resulting in a significant decrease after 6 months (1.34 ± 0.32 MDA nmol/ml) compared to baseline (1.50 ± 0.3 MDA nmol/ml, P = 0.002). The difference between the baseline and 6-month TBARS values was significantly lower in the Saf-tea group than the placebo group (P = 0.045 by ANCOVA).
Table 7 shows serum levels of AST, ALT, BUN, and creatinine in the study subjects before and during 6 months of supplementation in the placebo and Saf-tea groups. No differences were observed between the groups, time periods, or changes in the placebo and Saf-tea groups after 6 months of supplementation.
We evaluated the effectiveness and safety of safflower tea administered to postmenopausal women to attenuate osteoporosis and improve body antioxidant status. Drinking the safflower tea provided 72 mg of safflower polyphenols including isoflavones, lignans, and serotonin derivatives increased serum content of isoflavones and enterolactone, a major metabolite of lignans, and probably serotonin, although the latter was not measured. Supplementing with flaxseed, a rich source of enterolignan precursors (secoisolariciresinol and matairecinol) increases serum enterolactone concentrations [18] and the processing of flaxseed such as milling and crushing enhances enterolignan bioavailability [19] in humans. The intake of matairesinol, a major lignan in safflower tea, by the present study subjects was estimated to be approximately 10 mg per day, which was lower than that by flaxseed supplementation [18,19]. Matairesinol in the safflower tea was from a solvent extract, which appeared to be more easily available to the body than enterolignans from flaxseed powder. It is also noteworthy that the much smaller intake (~18 mg) of soy isoflavones included in safflower tea increased serum genestein levels compared to 70-200 mg soy isoflavone/day used in most supplementation studies [20-22]. This effect may be attributable to the serotonin derivatives (18 mg/day), one of the major polyphenolic components in the tea. However, the increases in body levels of isoflavones and enterolactone did not coincide with any improvements in the bone metabolic markers, although BMD appeared to remain the same in the safflower tea group compared to a decrease in the placebo group after 6 months of supplementation. The estrogenic bone protection activities of isoflavones and lignans have been repeatedly suggested and shown in a few animal and cell studies using isoflavones from soy and flaxseed but have rarely been confirmed in supplementation studies using humans. The main reason may be the amount of isoflavones or lignans used in the studies. When ovariectomized rats are fed defatted safflower powder [6] or ethanol extract [7], 4-4.4 mg of safflower polyphenols per 250 g of rat were observed, which is the equivalent of 800-880 mg per 50 kg body weight, whereas only 72 mg of safflower polyphenols were supplied to the present subjects who weighed 54-55 kg. Supplementing with isoflavones from soy and red-clover reduces bone loss after a 1 year intake of 43.5 mg/day [23] and increases BMD following six months of intake of 57-90 mg/day in postmenopausal women [24-26]. However, supplementing with 118 mg/day of isoflavones for 3 months does not have an effect on bone resorption markers [21], and 90 mg/day for 8 weeks or 96 mg/day for 9 months [27] does not improve BMD compared to placebo. Wu et al. [21] indicated that intervening with isoflavones (75 mg/day for 1 year) in postmenopausal Japanese women has a modest effect on BMD compared to the effects found in Westerners, and the effect was shown only at Ward's triangle. Lee et al. [28] reported that supplementation with three levels of isoflavones (100, 150, 200 mg/day) for 6 months appeared to reduce both serum osteocalcin and urinary excretion of DPD in postmenopausal women. Uesugi et al. [29] reported that 62.8 mg/day of isoflavones for 4 weeks reduced urinary DPD significantly and serum osteocalcin non-significantly in perimenopausal women. The effects of flaxseed lignans on bone health have been shown by a much a smaller number of human studies compared to those of soy isoflavones. Randomized control studies with either postmenopausal women [30-32] or adult men [32] did not show any effects on BMD and biomarkers following flaxseed supplementation. In contrast, Ward et al. [33] warned that feeding flaxseed secoisolariciresinol through a mother's milk may reduce bone strength in young rats. However, these results do not exclude the possibility of bone promoting effects of flaxseed. More discriminating research protocols are necessary to demonstrate the effects of components from flaxseed or other plants in different age groups and physiological states. The present study was the first human study on bone using tea containing safflower polyphenols in postmenopausal women with a matching placebo group. Although we were unable to attain definite positive bone health results, we found that the amount of tea used for the 6-month period of the present study was acceptable, judging from interviews with subjects. The safflower tea used in the present study had a considerable amount of serotonin derivatives (~18 mg), which is not found in soybeans, flaxseed, or Ssangwha extract. Serotonin aids bone formation in growing rats [34], and serotonin-abundant safflower seed powder has the same effect in rats with bone-fractures [2-5], as well as reducing bone loss in ovariectomized rats [6]. Therefore, it is worth attempting to use safflower seed products containing higher amounts of serotonin and phytoestrogens in human trials employing a crossover design to reduce inter-individual variations that seem to obscure statistical significance.
The safflower tea reduced peroxidation status in the body as shown by the reduction in serum TBARS levels. This was accompanied by an increase in body antioxidant capacity, as seen with higher levels of vitamins E, A, and C. Increases in serum vitamin concentrations were not related to changes in dietary intake of the Saf-tea and placebo groups after 6 months of supplementation. Therefore, the effects are regarded as being mainly from safflower tea polyphenols composed of flavones, lignin, and serotonin derivatives, which have radical scavenging activity and inhibit lipid peroxidation in in vitro [8,9] and in vivo [14] studies. Isoflavones have antioxidant activity [35]. Choi et al. [36] reported that supplementing with 200 mg of isoflavones/day for 12 weeks increases total antioxidant status both in peri- and post-menopausal women. Furthermore, Lee et al. [37] reported that 80 mg isoflavones/day decreases plasma malondialdehyde and increases total antioxidant status in hypercholesterolemic but not in normolipidemic postmenopausal women. However, they did not measure body antioxidant compounds individually. The flaxseed lignan secoisolariciresinol and its metabolites enterolactone and enterodiol exert antioxidant activity within in vitro systems [38,39]. Prasad [38] showed decreases in aortic malondialdehyde concentrations in rabbits fed a high cholesterol diet. In humans, dietary intake of 50 g of flaxseed for 4 weeks does not affect plasma hydroperoxide levels [40], whereas low serum enterodiol concentrations are associated with increased lipid oxidation [41]. The following studies with postmenopausal women failed to show antioxidant activity in humans supplemented with flaxseed for 4 weeks [42], or by a 6-week supplementation with 500 mg of secoisolariciresinol isolated from flaxseed [43]. In contrast, we found definite antioxidant effects with safflower tea containing 72 mg of safflower polyphenols, less than the amount used in the soy isoflavone and flaxseed lignan studies.
Our results showed that the safflower tea had strong antioxidant activity that helped reduce the incidence of degenerative disease in old age and has the potential to maintain bone health in postmenopausal women.
Figures and Tables
Fig. 1
Changes in serum cocnentrations of retinol, α-tocopherol and ascorbic acid of the subjects after three and six months of safflower tea supplementation. *,**Significantly different from baseline by paired t-test at P < 0.05 and P < 0.001. ***Significantly different from baseline by paired t-test at P < 0.001 and from placebo group by ANCOVA at P < 0.05.

Fig. 2
Changes in serum cocnentrations of TBARS of the subjects after three and six months of safflower tea supplementation. **Significantly different from baseline by paired t-test and from placebo group by ANCOVA at P < 0.05.

Table 3
Changes in daily energy, Ca and vitamins A, C, E intakes of the study subjects after six months of safflower tea supplementation

Table 5
Changes in serum genistein and enterolactone levels of the subjects after six months of safflower tea supplementation

References
1. Huh J. Herbe, Dongeui Bogam. 1989. Vol. 3. Seoul: Yeogang Publisher;2763.
2. Kim JH, Jeon SM, An MY, Ku SK, Lee JH, Choi MS, Moon KD. Effects of diet of Korean safflower (Carthamus tinctorius L.) seed powder on bone tissue in rats during the recovery of rib fracture. J Korean Soc Food Sci Nutr. 1998. 27:698–704.
3. Chung SY, Choi HJ, Chung MW, Ahn MR, Yoo TM, Rheu HM, Yang JS. Effects of safflower seed on the fracture healing in rat tibia. Yakhak Hoeji. 1999. 43:526–534.
4. Lee JY, Chang EJ, Kim HJ, Park JH, Choi SW. Antioxidative flavonoids from leaves of Carthamus tincotorius. Arch Pharm Res. 2002. 25:313–319.

5. Seo HJ, Kim JH, Kwak DY, Jeon SM, Ku SK, Lee JH, Moon KD, Choi MS. The effects of safflower seed powder and its fraction on bone tissue in rib-fractured rats during the recovery. Korean J Nutr. 2000. 33:411–420.
6. Kim HJ, Bae YC, Park RW, Choi SW, Cho SH, Choi YS, Lee WJ. Bone-protecting effect of safflower seeds in ovariectomized rats. Calcif Tissue Int. 2002. 71:88–94.

7. Cho SH, Choi SW, Choi YS, Kim HJ, Park YH, Bae YC, Lee WJ. Effect of ethanol extract of safflower seed on bone loss in ovariectomized rats. Food Sci Biotechnol. 2007. 16:392–397.
8. Kang GH, Chang EJ, Choi SW. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorious L.) seeds. J Food Sci Nutr. 1999. 4:221–225.
9. Kim EO, Oh JH, Lee SK, Lee JY, Choi SW. Antioxidant properties and quantification of phenolic compounds from safflower (Carthamus tinctorius L.) seeds. Food Sci Biotechnol. 2007. 16:71–77.
10. Kim EO, Kim KS, Lee WJ, Choi SW. Proliferative and differentiative effects of trachelogenin isolated from germinated safflower (Carthamus tinctorius L.) seeds on calvarial bone cells. Food Sci Biotechnol. 2009. 18:689–693.
11. Kim JH, Choi MS, Moon KD. Quality characteristic of drink and tea-bag processed with safflower seed powder. Korean J Postharvest Sci Technol. 2000. 7:171–176.
12. Kim JH, Park JH, Park SD, Choi SY, Seong JH, Moon KD. Preparation and antioxidant activity of health drink with extract powders from safflower (Carthamus tinctorius L.) seed. Korean J Food Sci Technol. 2002. 34:617–624.
13. Kim JH, Kim JK, Kang WW, Kim GY, Choi MS, Moon KD. Preparation of functional healthy drinks by ethanol extracts from defatted safflower seed cake. J Korean Soc Food Sci Nutr. 2003. 32:1039–1045.

14. Cho SH, Lee HR, Choi SW, Lee WJ, Choi Y. Formulated product containing safflower seed extract improves lipid status of ovariectomized rats. Korean J Gerontol. 2005. 15:39–46.
15. Jang JH, Yoon JY, Cho SH. Intake of dietary phytoestrogen and indices of antioxidant and bone metabolism of pre- and post-menopausal Korean women. Nutr Res Pract. 2007. 1:305–312.

16. Bieri G, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol in plasma or red blood cells by high pressure liquid chromatography. Am J Clin Nutr. 1979. 32:2143–2149.

17. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976. 15:212–216.

18. Tarpila S, Aro A, Salminen I, Tarpila A, Kleemola P, Akkila J, Adlercreutz H. The effect of flaxseed supplementation in processed foods on serum fatty acids and enterolactone. Eur J Clin Nutr. 2002. 56:157–165.

19. Kuijsten A, Arts IC, van't Veer P, Hollman PH. The relative bioavailbility of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr. 2005. 135:2812–2816.

20. Lee DH, Lee HS, Kim MH, Yoon ME, Sung CJ. Effects of isoflavones supplementation on bone mineral density and sex hormones in postmenopausal women. Korean J Nutr. 2002. 35:863–869.
21. Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, Fuku N, Teramoto T, Okuhira T, Ueno T, Uchiyama S, Urata K, Yamada K, Ishimi Y. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006. 55:423–433.

22. Dalais FS, Ebeling PR, Kotsopoulos D, McGrath BP, Teede HJ. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin Endocrinol (Oxf). 2003. 58:704–709.

23. Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA. The effects of phytoestrogen isoflavones on bone density in women: a double blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2004. 79:326–333.

24. Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW Jr. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998. 68:1375S–1379S.

25. Clifton-Bligh PB, Barber RJ, Fulcher GR, Nery ML, Moerton T. The effect of isoflavones extracted from red clover (Rimostil) on lipid and bone metabolism. Menopause. 2001. 8:259–265.

26. Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000. 72:844–852.

27. Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004. 11:290–298.

28. Lee BS, Won HJ, Lee SK, Choi Y, Yoon S, Park KH, Cho DJ, Song CH. The effect of isoflavone on serum lipid profiles and bone markers in postmenopausal women. J Korean Soc Menopause. 2002. 8:59–66.
29. Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: A four-week study. J Am Coll Nutr. 2002. 21:97–102.

30. Lucas EA, Wild RD, Hammond LJ, Khalil DA, Juma S, Daggy BP, Stoecker BJ, Arjmandi BH. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab. 2002. 87:1527–1532.

31. Dodin S, Lemay A, Jacques H, Légaré F, Forest JC, Mâsse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double-blind, wheat germ placebo-controlled clinical trial. J Clin Endocrinol Metab. 2005. 90:1390–1397.

32. Cornish SM, Chilibeck PD, Paus-Jennsen L, Biem HJ, Khozani T, Senanayake V, Vatanparast H, Little JP, Whiting SJ, Pahwa P. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl Physiol Nutr Metab. 2009. 34:89–98.

33. Ward WE, Ynan YV, Cheung AM, Thompson LU. Exposure to flaxseed and its purified lignan reduces bone strength in young but not older male rats. J Toxicol Environ Health A. 2001. 63:53–65.

34. Gustafsson BI, Westbroek I, Waarsing JH, Waldum H, Solligård E, Brunsvik A, Dimmen S, van Leeuwen JP, Weinans H, Syversen U. Long-term serotonin administration leads to higher bone mineral density, affects bone architecture, and leads to higher femoral bone stiffness in rats. J Cell Biochem. 2006. 97:1283–1291.

35. Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010. 8:89–98.

36. Choi Y, Yoon S, Lee MJ, Lee SK, Lee BS. Dose response relationship of isoflavone supplementation on plasma lipid profiles and total antioxidant status in perimenopausal and postmenopausal women. Korean J Nutr. 2001. 34:322–329.
37. Lee JH, Kim EM, Chae JS, Jang YS, Lee JH, Lee G. The effect of isoflavone supplement on plasma lipids and antioxidant status in hypercholesterolemic postmenopausal women. Korean J Nutr. 2003. 36:603–612.
38. Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation. 1999. 99:1355–1362.

39. Niemeyer HB, Metzler M. Differences in the antioxidant activity of plant and mammalian lignans. J Food Eng. 2003. 56:255–256.

40. Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ. Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr. 1995. 61:62–68.

41. Vanharanta M, Voutilainen S, Numi T, Kaikkonen J, Roberts LJ, Morrow JD, Adlercreutz H, Salonen JT. Association between low serum enterolactone and increased plasma F2-isoprostanes, a measure of lipid peroxidation. Atherosclerosis. 2002. 160:465–469.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download