Abstract
The precise effects of protein intake on fractional synthesis rate (FSR) of muscle protein are still under debate. The sample size of these studies was small and the conclusions in young and elderly subjects were inconsistent. To assess the effect of dietary protein intake on the FSR level, we conducted a meta-analysis of controlled protein intake trials. Random-effects models were used to calculate the weighted mean differences (WMDs). Ten studies were included and effects of short-term protein intake were evaluated. In an overall pooled estimate, protein intake significantly increased the FSR (20 trials, 368 participants; WMD: 0.025%/h; 95%CI: 0.019-0.031; P < 0.0001). Meta-regression analysis suggested that the protein dose was positively related to the effect size (regression coefficient = 0.108%/h; 95%CI: 0.035, 0.182; P = 0.009). A subgroup analysis indicated that protein intake significantly increased FSR when the protein dose was ≤ 0.80 g/kg BW (16 trials, 308 participants; WMD: 0.027%/h; 95%CI: 0.019-0.031; P < 0.0001), but did not affect FSR when the protein dose was > 0.80 g/kg BW (4 trials, 60 participants; WMD: 0.016%/h; 95%CI: 0.004-0.029; P = 0.98). In conclusion, this study is the first integrated results showing that a short-term protein intake is effective at improving the FSR of muscle protein in the healthy elderly as well as young subjects. This beneficial effect seems to be dose-dependent when the dose levels of protein range from 0.08 to 0.80 g/kg BW.
Aging is accompanied by changes in body composition with a progressive loss of muscle mass, strength, and metabolic function [1-4]. A net loss of muscle with aging is the result of a chronic imbalance between muscle protein synthesis and breakdown. The age-related loss of muscle protein synthesis is referred as sarcopenia [5-9]. Chronic muscle loss is estimated to affect 30% of people older than 60 y and may affect > 50% of those older than 80 y [9-12]. Sarcopenia is associated with a 3- to 4-fold increased likelihood of disability, which in turn is associated with substantial socioeconomic and health care spending. One analysis estimated that in 2000, sarcopenia was responsible for $18.5 billion in health care costs [9,12].
Muscle plays a central role in whole-body protein metabolism [4]. Muscle protein metabolism alternates between periods of net catabolism in the postabsorptive state and net anabolism in the postprandial states, with the latter being primarily a result of changes in muscle protein synthesis [13,14]. Protein or amino acids are potent stimulators of muscle protein synthesis in both the young and elderly [14,15,16-24]. Many reports suggest that protein or amino acids may be useful at improving the fractional synthesis rate (FSR) of muscle protein in essential tissues and organs of human body [4,8,9,15,25-31]. Above all, it is important to supply a source of shortage protein, which increases the muscle mass and strength through protein synthesis.
However, a study of net balance was found to be similar in young and elderly men and could not explain the loss of muscle that occurs with age [32]. No studies have shown the integrated results about the FSR of muscle protein directly in a leg muscle. Because the sample size of these studies was small, it is difficult to compare with the level of FSR. As a result, the precise effects of protein or amino acids ingestion are still under debate.
Therefore, we firstly used FSR level of muscle protein measured by the same method. The level of FSR was calculated by measuring the direct incorporation of phenylalanine or leucine into protein by using the precursor-product model [33]. The aim of the present study was to identify and combine ten published randomized controlled studies that investigated the effects of protein or amino acids intake on FSR.
We systematically searched PubMed, reviews, and reference lists of relevant papers with the strategy of using the term "fractional synthesis rate OR muscle protein synthesis OR muscle protein anabolism" paired with the following: "dietary protein," "high protein diet," "essential amino acid," and "amino acid."
Searches for English language studies that were published between 1 January 1976 and 6 July 2009 were performed in PubMed. We selected peer reviewed papers investigated the effects of dietary protein on FSR. This selection excluded studies without measuring FSR (%/h) in muscle protein. The fractional synthetic rate (FSR) of muscle protein was evaluated by estimating the direct incorporation of phenylalanine or leucine into protein to examine amino acid kinetics in the vastus lateralis muscle by using the precursor-product model, which was measured before and after the ingestion of protein in elderly or young subjects [33]. Participants must have been treated with dietary protein for 6 h. Trials evaluating the FSR of muscle protein with other methods were also included for systematic review but were not selected for meta-analysis, because these data could not be combined and compared quantitatively.
All literature searches were independently reviewed by 3 authors to identify relevant studies that met the inclusion criteria as part of our quality-control process. We extracted only the studies including FSR measurement in muscle protein. Disparities about qualitative information and quantitative data from each study were resolved by discussion. Data on study size, population characteristics (age, sex and year), treatment regimen (dose of protein and duration of treatment), and change in FSR levels were extracted.
Our primary outcome was the net change in FSR of muscle protein due to dietary protein ingestion, which was calculated using the DerSimonian-Laird method for a random-effects model [34]. Random-effects models were used to calculate the weighted mean differences (WMDs), 95% CIs, and corresponding P values for heterogeneity. The estimates of the individual studies were weighted based on the inverse of the variance, which is related to the sizes of the study populations. Between-study heterogeneity of treatment effects was assessed using Cochran's Q test (P < 0.1). The I2 statistic was also examined, and we considered I2 > 50% to indicate significant heterogeneity between the studies [35]. Previously defined subgroup analyses and meta-regression analyses were performed to explore heterogeneity in effects and the influence of study characteristics. Publication bias was assessed visually by examining a funnel plot as a function of effect size, Egger regression test [36], and failsafe N test [37]. Statistical analyses were performed with MIX software (version 1.7; LEON BAX) and SPSS software (version 12; SPSS Inc. Chicago).
A total of 3,644 articles were identified in a search of the PubMed and from the reference list of the selected articles. Of these, 3,622 articles were excluded because they did not meet the inclusion criteria or their interventions were not relevant to the purpose of this meta-analysis (such as vitamin C or other nutrients intended to improve muscle protein). Full text assessment of the 22 potentially relevant articles was identified. The most common reasons for exclusion were as follows: protein was not admonistered orally but intravenous or intra-arterial infusion in 2 studies [32,38] and that levels of fractional synthesis rate (FSR) were not reported in 10 studies [28,29,39-46]. Ten articles [14,17-24,47] were selected for the meta-analysis and were considered by us to meet all the eligibility criteria (Fig. 1).
We identified 10 articles [14,17-24,47] with 20 trials and 368 subjects for inclusion in our study. Characteristics of the studies included in the analysis are shown in Table 1. The studies varied in size from 6 to 24 subjects. As for the 20 studies that evaluated the FSR of muscle protein, 10 trials [17,19-22] investigated the effect of protein ingestion consisting of essential amino acids in healthy elderly and young subjects. The other 10 trials investigated the effect of protein ingestion consisting of various amino acids in healthy elderly and young individuals [18,21-24,47]. The average age of the participants varied from 20 to 73 y. Doses of protein in the included studies ranged from 0.12 to 1.13 g/kg of body weight during trials, and the treatment duration varied from 3 h to 6 h. Protein was ingested to one meals in the study period of 11 trials [14,17,20,21,23], and consumed as small liquid meals or boluses which divided by periods (12 to 18 times) in 9 trials [18,19,22,24,47].
Twenty trials evaluated the effect of short-term protein ingestion on FSR level of muscle protein. The work of Paddon-Jones et al. [21] was separated into 2 trials (effect of muscle protein synthesis in elderly human following isocaloric ingestion of amino acids or whey protein) for analysis. Because one study [24] reported as a unit of percent of FSR per day, the data was transformed as the unit per hour. Two studies [24,47] reported FSR level of the protein ingestion and exercise. One study reported FSR level of 2.5 g to 40 g essential amino acids (EAAs). We extracted the FSR data from 10 g EAAs for meta-analysis [20].
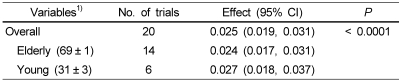
First, the data were pooled from 10 studies. FSR levels were significantly higher in the protein ingested subjects than in the control ingested subjects (20 comparisons, 368 participants; WMD: 0.025%/h; 95%CI: 0.019-0.031; P < 0.0001) (Fig. 2). Significant heterogeneity for this outcome was found (heterogeneity Q = 1253, I2 = 98%, P < 0.0001). The FSR level of the healthy elderly and young subjects is shown in Table 2. When studies of elderly and young subjects on the FSR of muscle protein were analyzed, we determined that the FSR level was 0.024%/h (95% CI, 0.017-0.031; P < 0.0001) in the elderly subjects (14 comparisons, 274 participants) and 0.027%/h (95%CI, 0.018-0.037; P < 0.001) in the young subjects (6 comparisons, 94 participants). FSR levels were higher in the young subjects (13%) than in the elderly subjects (Table 2).
The sources of heterogeneity were investigated by meta-regression methods. Meta-regression analysis of the data showed that the protein dose of FSR was positive related to effect size (regression coefficient = 0.108%/h; 95%CI: 0.035, 0.182; P = 0.009), which explained significant heterogeneity of the effect. The study duration (range: 3~6 h/ test period), and the average age of the participants were not effect modifiers.
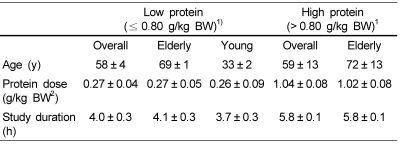
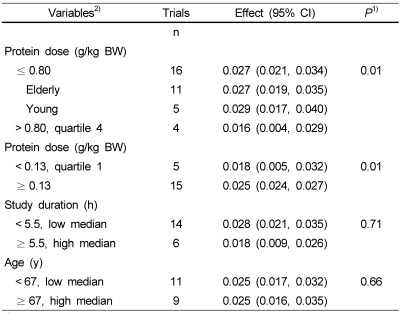
Subgroup analyses were done in order to identify sources of heterogeneity. Rand and colleagues proposed that the recommended dietary allowance for protein for all men and women aged 19 years and older is 0.8 g/kg/day [48]. Because of the lack of reference ranges for protein dose, we predefined the first to third quartiles as low protein dose group (≤ 0.80 g/kg BW) for all included trials, whereas the fourth quartile (> 0.80 g/kg BW) was assigned as the high group. Characteristics of the subgroups are shown in Table 3. Protein intake significantly increased the level of FSR when the protein dose was ≤ 0.80 g/kg BW (16 trials, 308 participants; WMD: 0.027%/h; 95%CI: 0.019-0.031; P < 0.0001), but had nonsignificant effect on FSR if the protein dose was > 0.80g/kg BW (4 trials, 60 participants; WMD: 0.016%/h; 95%CI: 0.004-0.029; P = 0.98) (Table 4). When effect size of low protein dose on the FSR of muscle protein was analyzed in elderly and young subjects, we determined that the FSR level was 0.027%/h in the elderly subjects (11 comparisons, 230 participants) and 0.029%/h in the young subjects (5 comparisons, 78 participants). FSR levels were not different between the young and elderly subjects (7% difference). The FSR level of muscle protein was effective when protein dose was low, as well as the trend was also statistically significant when < 0.13 g/kg BW (cutoff for first quartile) was used as cutoffs or thresholds for the subgroup analysis. The FSR was continuously increased until the amount of protein dose was 0.08 to 0.80 g/kg BW (Table 4).
A sensitivity analysis according to other participant characteristics and study design features found that there were no effects due to mean age of the study population, or study duration on the estimated change in FSR of muscle protein due to protein ingestion (Table 4).
Funnel plot had the expected asymmetric funnel shape (Fig. 3 and 4). Egger test for publication bias (P = 0.72) indicated that there was little or no publication bias in our analysis. A statistical analysis of the dietary protein intake and the post-exercise did not suggest any significant publication bias (Egger test, P = 0.95 for subgroup of the protein intake; Egger test, P = 0.45 for subgroup of the post-exercise; Egger test, P = 0.97 for subgroup of low protein dose). A fail-safe N test indicated that it would take 8096 (for all studies), 8096 (for the dietary protein intake subgroup), or 5794 (for low protein dose subgroup) unpublished studies that would be required to change the results to a nonsignificant effect.
Compared with control, short-term protein ingestion resulted in a substantial improvement in FSR level of muscle protein. Overall effect size of FSR was higher in the young subjects than in the elderly, but that of protein intake was not different between the young and elderly subjects. There was significantly heterogeneity between studies in the overall analysis. Therefore, we considered that the characteristics of subjects and study design may have influenced the results of these studies. An evidence from meta-regression suggested that the heterogeneity was explained by the protein dose, and indicated that protein dose was positively related to the effect size. The FSR was continuously increased until amount of protein dose was 0.08 to ≤ 0.80 g/kg BW (Table 4). In other words, as protein dose increased to > 0.80 g/kg BW, the level of FSR was gradually attenuated.
Our subgroup analysis showed that the level of FSR was increased when protein dose was low, but decreased when protein dose was high. These data suggest that the protein ingestion was able to improve the muscle protein synthesis in both the healthy elderly and young individuals. The consistent results identified by the meta-regression and subgroup analysis enhance confidence of the contrasting results in the different subgroups and suggest that protein dose largely influenced the level of FSR.
The age-related loss of muscle cells and strength occurs at a rate of approximately 10% per decade after 50 years of age [45]. A decline of skeletal muscle mass with aging is attributed to a disruption in regulation of muscle protein synthesis and/or breakdown [16]. Recent studies [29,41,44,46] have shown that dietary protein or amino acids ingestion improves muscle protein synthesis, which is similar between young and old men; however, the response is delayed with aging. Specifically, inappropriate or insufficient protein intake in the elderly did not induce the muscle protein synthesis. Further, inappropriate or insufficient nutritional intake may impair anabolic signaling in skeletal muscle tissue, which may be a key factor of sarcopaenia [1,2,8,20,31]. More recent study [49] has shown that the phosphorylation of p70 ribosomal S6 kinase (p70S6K) and eukaryotic initiation factor 4E binding protein 1 (4EBP1) was blunted in older men. Both p70S6K and 4EBP1, downstream effectors of mTOR signaling, are key intracellular pathway coordinating signals in the regulation of protein synthesis. They play a key regulatory role in the regulation of translation initiation [50]. Drummond et al. [41] suggested that unresponsive ERK1/2 signaling and AMPK activation in old muscle may be playing a role in the delayed activation of muscle protein synthesis. The mechanism whereby protein intake may improve FSR, especially when protein dose is low, remains uncertain.
It has been reported that muscle from elder individuals is resistant to the anabolic effects of amino acids. Wolfe and coworkers have extensively investigated that muscle protein synthesis is stimulated in young and elder subjects when protein or EAAs are ingested [14,17,19,29,51]. Paddon-Jones et al. [17] have shown that the ingestion of essential amino acids (EAA) stimulates muscle protein synthesis to a similar extent in young and old individuals. Aged muscle has a decreased sensitivity and responsiveness to amino acids at physiological concentrations but can still respond if the increase of aminoacidaemia is large enough [17,19]. It has recently reported that among amino acids, branched chain amino acids and especially leucine supplementation improve muscle protein synthesis in elderly men independently of hyperaminoacidaemia [18]. Symons et al. [23] clearly shows that aging does not impair the ability to acutely synthesize muscle protein after ingestion of a common protein-rich food.
However, recent study has reported that long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men [40]. Koopman et al. [47] showed that co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Welle et al. [24] also showed that high-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62 to 75-yr-old men and women.
These different results can be due to the quantity and the quality of ingested protein. Specifically, in the elderly, inappropriate or insufficient protein intake may impair anabolic signaling in skeletal muscle tissue [1,2,8,20,31]. It has been shown that aging is not associated with an ability of skeletal muscle to respond to low doses (~7.5 g) of EAAs whereas higher doses (10-15 g) are able to stimulate muscle protein synthesis to a similar extent as the young [17,21,31]. Further, the proportional FSR/EAA dose-response is responsible for stimulating muscle protein synthesis while the supplementation of non-essential amino acids does not further enhance the anabolic stimulus. It is important to supply leucine to small bolus of EAAs in enhancing the ability of aged muscle. Rieu et al. [18] reported that leucine-supplemented meals may improve or normalize muscle protein synthesis in aging muscle. Katsanos et al. [14] has shown that addition of supplemental leucine to a bolus of EAAs in aged humans increases muscle protein synthesis. A repeated supplementation of EAAs at short-term can improve muscle protein synthesis and prevent a loss of muscle protein with aging. Consequently, dietary supplementation with sufficient EAAs alone may provide a practical and efficient means of stimulating protein synthesis in the elderly.
In the other hand, the FSR in this study was continuously increased until amount of protein dose was 0.08 to ≤ 0.80 g/kg BW (Table 4). The Estimated Average Requirement (EAR) of 0.66 g protein/kg body weight/d and the Recommended Dietary Allowance (RDA) 0.80 g protein/kg body weight/d are deemed the same for all apparently healthy men and women aged ≥ 19 y [52]. However, it has not explained whether the RDA of 0.80 g protein/kg body weight/d in the elderly is adequate. Level of FSR in this study is well consistent with level of the RDA in the elderly.
There are distinct differences in protein response between the young and the elderly. These differences can be the result of changes in signaling pathways over the course of decades. In line with this, there are also indications that protein digestion and absorption rates are different between the young and the old. These findings provide indirect evidence suggesting that the efficiency of protein synthesis after a meal may be impaired in older populations. Gut physiology and nutritional status in the elderly can be changed by the rate of gastric emptying and first-pass splanchnic uptake [53]. Symons et al. [23] has shown that in comparison with the peak concentrations of the more rapidly digested and absorbed amino acids or whey protein supplements, peak concentrations of AAs in both young and elder groups occurred more slowly after beef ingestion. This difference may be due to the smaller body size (lean and total mass) of the elderly than of the young subjects, consequently representing a proportionally greater nutrient intake for physically smaller subjects. One study [43] indicated that a short-term high-protein diet does not augment muscle protein synthesis. Because subjects with a high protein dose may already have the amount of sufficient muscle protein synthesis, the remained protein transformed to a fuel for energy production [15,32]. Notwithstanding, ingestion of essential amino acid (EAA) results in a change from net muscle protein degradation to net muscle protein synthesis after heavy resistance exercise [39,41,42]. One study [28] documented that branched-chain amino acid supplementation to soy protein enhances whole-body protein synthesis in patients with chronic wasting diseases (eg, chronic obstructive pulmonary disease), but not in the healthy elderly. Furthermore, protein source (e.g. whey, casein, vegetable or animal) and type (intact, hydrolyzed or specific peptide sequences) can result in different responses in young and old subjects [17,21-23,29-31]. This difference is considered to be of high biological value according to source of dietary protein. Ingestion of higher quality of protein or amino acids, as well as higher quantity of EAAs or leucine with EAAs is a good method to prevent muscle loss in the elderly.
Thus, the continuous sufficient protein intake or the combination of resistance exercise and protein ingestion should be a useful strategy to improve the physical function of aging muscle through muscle protein synthesis. We suggest that protein ingestion can be a good alternative to combat muscle loss. Ultimately, this result appears to support the idea that we should provide sufficient protein to the elderly as well as all individuals who would need muscle protein synthesis. In the near future, long-term intervention studies should be warranted to address the efficacy of protein and/or amino acids supplementation as an interventional strategy to attenuate the loss of skeletal muscle mass with ageing.
The present study had several potential limitations. First, only 4 trials were combined for analysis in the high protein dose group. When the high protein dose (> 0.80 g/kg BW) was used as cutoffs for subgroup analysis, the lack of statistical significance might be due to the small number of studies. Second, because significant heterogeneity was observed in the effect size of all studies and in the subgroup with low protein dose, both funnel plots and Egger tests did not provide sufficient evidence against a publication bias. However, a fail-safe N test indicated that it would take 8096 (for the dietary protein intake subgroup), or 5794 (for low protein dose subgroup). The existence of that many unpublished studies is improbable; hence, this fail-safe N added greatly to the confidence of the results of our meta-analysis. Third, the mean study duration and mean age were statistically different between groups with high and low protein intakes (Table 4) and varied largely in all studies. The potential influence of these factors was estimated by the sensitivity analysis and the meta-regression analysis, which indicated that the mean study duration (range: 3-6 h) and the average age of the participants were not effect modifiers. It is an interesting point that the FSR was effective when the need of additional protein (≤ 0.80 g/kg BW) for muscle protein synthesis was accepted, which suggests that daily protein intakes may be enough to induce a favorable effect in the elderly. Other explanations may be that the sample size was not large enough, which resulted in a lack of power to detect the effect. Thus, additional studies are needed.
This meta-analysis suggests that the FSR level of muscle protein is likely to be benefitted from short-term protein intake in the healthy young as well as elderly subjects. This beneficial effect seems to be dose-dependent when the dose of protein ranges from 0.08 to 0.80 g/kg BW. Future research should focus on greater quantity trials with longer follow-ups to support the certainty regarding the clinical effects.
References
1. Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci. 1995; 50:1–4. PMID: 7493199.

2. Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997; 127:998S–1003S. PMID: 9164283.
3. Roberts SB. Effects of aging on energy requirements and the control of food intake in men. J Gerontol A Biol Sci Med Sci. 1995; 50:101–106. PMID: 7493200.
4. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006; 84:475–482. PMID: 16960159.

5. Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavychain and sarcoplasmic protein in humans. Am J Physiol. 1997; 273:E790–E800. PMID: 9357810.
6. Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993; 264:E693–E698. PMID: 8498491.

7. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am J Physiol. 1999; 277:E118–E125. PMID: 10409135.

8. Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sacopenia of aging: Underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009; 35:28–35. PMID: 19319843.
9. Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sacopenia of aging. Am J Clin Nutr. 2008; 87:1562S–1566S. PMID: 18469288.
10. Baumgartner RN, Koehler KM, Gallagher D. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998; 147:755–763. PMID: 9554417.

11. Aniansson A, Sperling L, Rundgren A, Lehnberg E. Muscle function in 75-year-old men and women. A longitudinal study. Scand J Rehabil Med Suppl. 1983; 9:92–102. PMID: 6585946.
12. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004; 52:80–85. PMID: 14687319.
13. Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond). 1982; 63:519–523. PMID: 6181926.

14. Katsanos CR, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006; 291:E381–E387. PMID: 16507602.

15. Borsheim E, Bui QUT, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008; 27:189–195. PMID: 18294740.
16. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004; 286:E92–E101. PMID: 14506079.

17. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004; 286:E321–E328. PMID: 14583440.

18. Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006; 575:305–315. PMID: 16777941.

19. Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999; 277:E513–E520. PMID: 10484364.
20. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005; 19:422–424. PMID: 15596483.

21. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006; 41:215–219. PMID: 16310330.

22. Koopman R, Verdijk L, Manders RJF, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJM, van Loon LJC. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006; 84:623–632. PMID: 16960178.

23. Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007; 86:451–456. PMID: 17684218.

24. Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998; 274:E677–E683. PMID: 9575829.
25. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009; 89:161–168. PMID: 19056590.

26. Caso G, Feiner J, Mileva I, Bryan LJ, Kelly P, Autio K, Gelato MC, McNurlan MA. Response of albumin synthesis to oral nutrients in young and elderly subject. Am J Clin Nutr. 2007; 85:446–451. PMID: 17284742.
27. Bos C, Benamouzig R, Bruhat A, Roux C, Mahé S, Valensi P, Gaudichon C, Ferrière F, Rautureau J, Tomé D. Short-term protein and energy supplementation activates nitrogen kinetics and accretion in poorly nourished elderly subjects. Am J Clin Nutr. 2000; 71:1129–1137. PMID: 10799375.

28. Engelen MPKJ, Rutten EPA, De Castro CLN, Wouters EFM, Schols AMWJ, Deutz NEP. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007; 85:431–439. PMID: 17284740.

29. Katsanos CS, Chinkes DL, Paddon-Jones D, Xiao-jun Zhang X, Aarsland A, Robert R, Wolfe RR. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008; 28:651–658. PMID: 19083472.

30. Wilkinson SB, Tarnopolsky MA, MacDonald MJ, MacDonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007; 85:1031–1040. PMID: 17413102.

31. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005; 82:1065–1073. PMID: 16280440.

32. Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001; 286:1206–1212. PMID: 11559266.

33. Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2005. New Jersey: John Wiley & Sons Inc.;p. 9–76.
34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188. PMID: 3802833.

35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.

36. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID: 9310563.

37. Rosenthal R. The "file drawer problem" and tolerance for null results. Psychol Bull. 1979; 86:638–641.

38. Donatelli F, Schricker T, Parrella P, Asenjo F, Wykes L, Carli F. Intraoperative infusion of amino acids induces anabolism independent of the type of anesthesia. Anesth Analg. 2006; 103:1549–1556. PMID: 17122238.

39. Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999; 276:E628–E634. PMID: 10198297.
40. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WKWH, Dendale P, van Loon LJC. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009; 89:1468–1475. PMID: 19321567.

41. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008; 104:1452–1461. PMID: 18323467.

43. Walrand S, Short KR, Bigelow ML, Sweatt AJ, Hutson SM, Nair KS. Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab. 2008; 295:E921–E928. PMID: 18697911.

44. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Health ABC Study. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008; 87:150–155. PMID: 18175749.

45. Roth E. Skeletal muscle gain: how much can be achieved by protein and amino acid administration? Curr Opin Clin Nutr Metab Care. 2008; 11:32–33. PMID: 18090655.

46. Lord C, Chaput JP, Aubertin-Leheudre M, Labonté M, Dionne IJ. Dietary animal protein intake: association with muscle mass index in older women. J Nutr Health Aging. 2007; 11:383–387. PMID: 17657359.
47. Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJM, Kuipers H, van Loon LJC. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008; 99:571–580. PMID: 17697406.

48. Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003; 77:109–127. PMID: 12499330.

49. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie M. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009; 587:211–217. PMID: 19001042.

50. Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006; 21:362–369. PMID: 16990457.

51. Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002; 6:358–362. PMID: 12459885.
52. Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr. 2008; 88:1322–1329. PMID: 18996869.
53. O'Mahony D, O'Leary P, Quigley EM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002; 19:515–527. PMID: 12182688.
Fig. 1
Flow chart representing the publication selection process. Flow chart shows the number of citations retrieved by individual searches and the number of trials included in the review. FSR, Fractional synthesis rate.
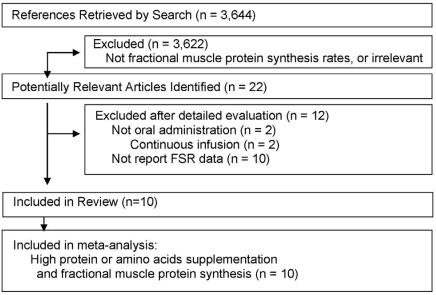
Fig. 2
Random-effect meta-analysis of weight mean differences (95% CI) in FSR of muscle protein with dietary protein intake compared with control. Sizes of data markers indicate the weight of each study in the analysis.
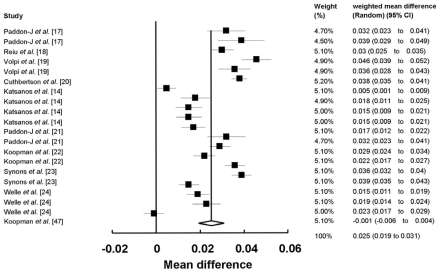
Fig. 3
Funnel plot of all individual studies in the meta-analysis. Studies that evaluated the effect of protein ingestion on FSR were plotted with mean difference on the horizontal axis and the inverse standard error along the vertical axis.
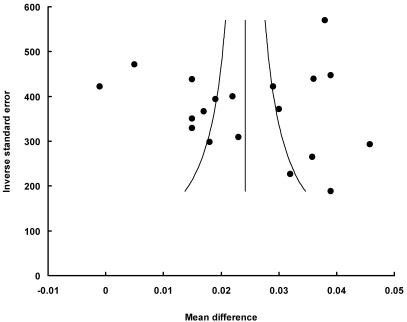
Fig. 4
Funnel plot of studies in the meta-analysis with the FSR level (≤ 0.80 g) of low protein dose. Studies that evaluated the effect of protein ingestion on FSR were plotted with mean difference on the horizontal axis and the inverse standard error along the vertical axis.
Table 1
Characteristics of observational studies that evaluated dietary protein intake in the included trials
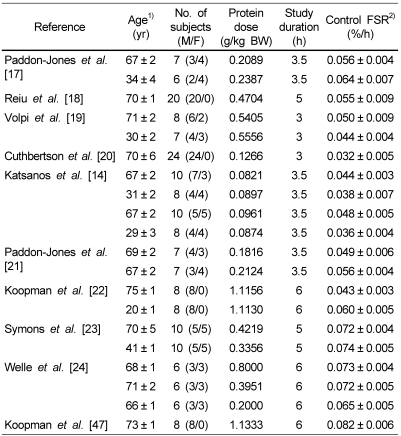
Table 2
Summary of the results on the association of dietary protein intake on FSR level in the meta-analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download