Abstract
Figures and Tables
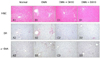 | Fig. 1
Histological analysis of liver sections. (A) Normal. (B) DMN (10 mg/kg per day for 3 consecutive days per week for 4 weeks) alone. (C) DMN with 10% grape skins. (D) DMN with 10% grape seeds. The sections were stained with hematoxylin-eosin (H&E) and with Sirius red (SR). Activated HSCs were detected by immunohistochemistry with α-SMA antibody (α-SMA). |
Table 1

1) Contained per kg : Vitamin A, 13,800 IU; Vitamin D3, 2,584IU; Vitamin E, 65 mg; Thiamine, 0.72 ppm; Riboflavin, 1.96 ppm; Pantothenate, 7.47 ppm; Biotin, 0.32 ppm; Folic acid, 0.76 ppm; Niacine, 15.3 ppm; Pyridoxine, 6.3 ppm; Choline, 1,002 ppm
2) Contained per kg : Cu, 28.4 mg; Fe, 39.2 mg; Zn, 74.6 mg; Mn, 77.7 mg; Se, 0.4 mg; I, 0.9 mg
Table 2

DMN was intraperitoneally given at 10 mg/kg on 3 consecutive days per week for 4 weeks to each group except the normal group.
DMN, DMN alone; K10, 10% diet of grape skin; S10, 10% diet of grape seed; DK10, 10% diet of grape skin with DMN; DS10, 10% diet of grape seed in with DMN
Values are the mean ± SE of rats.
Statistical significance: *P < 0.05 vs. DMN, and #P < 0.05, ##P < 0.01 vs. normal, respectively
Table 3

DMN was intraperitoneally given at 10 mg/kg on 3 consecutive days per week for 4 weeks to each group except normal group.
DMN, DMN alone; K10, 10% diet of grape skin; DK10, 10% diet of grape skin with DMN; S10, 10% diet of grape seed; DS10, 10% diet of grape seed with DMN
Values are the mean ± SD of rats.
Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMN, and #P < 0.05, ##P < 0.01, ###P < 0.001 vs. normal, respectively
AST, aspartate transaminase; ALT, alanine transaminase
Table 4

DMN was intraperitoneally given at 10 mg/kg on 3 consecutive days per week for 4 weeks to each group except the normal group.
Values are the mean ± SE of 6 rats.
Statistical significance: ***P < 0.001 vs. DMN, and ###P < 0.001 vs. normal, respectively
DMN, DMN alone; K10, 10% diet of grape skin without DMN; DK10, 10% diet of grape skin with DMN; S10, 10% diet of grape seeds without DMN; DS10, 10% diet of grape seeds with DMN
Table 5

DMN was intraperitoneally given at 10 mg/kg on 3 consecutive days per week for 4 weeks to each group except normal group.
DMN, DMN alone; K10, 10% diet of grape skin without DMN; DK10, 10% diet of grape skin with DMN; S10, 10% diet of grape seeds without DMN; DS10, 10% diet of grape seeds with DMN
Values are the mean ± SD of rats. Liver samples were hydrolyzed and assayed for hydroxyproline.
Statistical significance: **P < 0.01 vs. DMN, and ###P < 0.001 vs. normal, respectively
Table 6

DMN was intraperitoneally given at 10 mg/kg on 3 consecutive days per week for 4 weeks to each group except normal group.
DMN, DMN alone; K10, 10% diet of grape skin without DMN; DK10, 10% diet of grape skin with DMN; S10, 10% diet of grape seeds without DMN; DS10, 10% diet of grape seeds with DMN.
Values are the mean ± SD of rats.
Liver samples were hydrolyzed and assayed for MDA.
Statistical significance: *P < 0.05, **P < 0.01 vs. DMN, and ##P < 0.01 vs. normal, respectively




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download