Abstract
Blueberry was enzymatically hydrolyzed using selected commercial food grade carbohydrases (AMG, Celluclast, Termamyl, Ultraflo and Viscozyme) and proteases (Alcalase, Flavourzyme, Kojizyme, Neutrase and Protamex) to obtain water soluble compounds, and their protective effect was investigated against H2O2-induced damage in Chinese hamster lung fibroblast cell line (V79-4) via various published methods. Both AMG and Alcalase hydrolysates showed higher total phenolic content as well as higher cell viability and ROS scavenging activities, and hence, selected for further antioxidant assays. Both AMG and Alcalase hydrolysates also showed higher protective effects against lipid peroxidation, DNA damage and apoptotic body formation in a dose-dependent fashion. Thus, the results indicated that water soluble compounds obtained by enzymatic hydrolysis of blueberry possess good antioxidant activity against H2O2-induced cell damage in vitro.
Oxygen is essential for metabolic processes in the body. However, oxygen derived free radicals known as reactive oxygen species (ROS) are reported to exert detrimental effects, such as membrane lipid peroxidation, alteration of lipid-protein interactions, enzyme inactivations, and DNA breakage [1]. ROS have been implicated in initiating, accompanying or causing pathogenesis of many diseases [2]. The abnormalities in the configuration of lipid compounds may cause the overproduction of ROS. Antioxidant enzyme activities and lipid peroxidation were increased consequences of those reactions which may be related to the pathophysiology of depression [3]. Further, H2O2 is thought to be the major precursor of highly reactive free radicals such as hydroxyl radicals (HO.), which has been reported to induce apoptosis in the cells of the central nervous system [4].
A great deal of interest has been increased on plant research during last decade all over the world, and a large body of evidence has revealed the immense potential of plants used in traditional medicinal treatment. Various medicinal plants have been identified and studied for their potential to be used as medicinal formulations in pharmacology [5]. Chemoprevention has been referred to the prevention of cancer via specific constituents to alleviate or minimize the carcinogenic process [6]. Most of the extracts from plants are known to exert their effect by antioxidant mechanisms either quenching ROS, inhibiting lipid peroxidation or stimulating cellular antioxidant defenses [7].
A higher antioxidant capacity has been reported in blueberry that can neutralize free radicals which cause neurodegenerative disease, cardiovascular disease, and cancer [8,9]. Further, blueberry is a particularly nutritious fruit that provides benefits for a wide variety of conditions. Many studies have proven that the consumption of blueberry shows protective effects in relation to many diseases, such as reduction of coronary heart disease, treatment of urinary tract disorders, retarding brain aging and anticarcinogenic activity [10]. In addition, blueberry intake has shown improved digestion, reduction of colon inflammation and promotion of urinary tract health [11]. Genus Vaccinium is known to possess anti-diabetic activity and has been used in traditional medicine, especially for the secondary complications of diabetes [12]. The aims of this study were to obtain water soluble compounds from blueberry by food grade digestion enzymes and to evaluate their protective effect against H2O2-induced damage in Chinese hamster lung fibroblast (V79-4) cell line.
Blueberry was collected from a farm of Jeju Nong San Co. Ltd. in Korea.
Carbohydrases such as Viscozyme L (ability to liberate bound materials and to degrade non-starch polysaccharides), Celluclast 1.5 L FG (catalyzes the breakdown of cellulose into glucose, cellobiose and higher glucose polymers), AMG 300 L (hydrolyzes 1,4-and 1,6-α-linkages in liquefied starch), Termamyl 120 L (hydrolyses 1,4-α-glucosidic linkages in amylose and amylopectin), Ultraflo L (breakdown of β-glucans, pentosans and other gums) and proteases such as Protamex (production of non-bitter protein hydrolysis), Kojizyme 500 MG (amino and carboxy peptidase activities), Neutrase 0.8 L (endopeptidase activities), Flavourzyme 500 MG (endoprotease and exopeptidase activities), Alcalase 2.4 L (endopeptidase activity) were purchased from Novo Co. (Novozyme Nordisk, Bagsvaed, Denmark). Optimum hydrolysis conditions and characteristics of the enzymes were described in Table 1. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL, (Paisley, UK). All other chemicals used were in analytical grade.
The method described by Heo et al. [13] was used with slight modifications to prepare the enzymatic hydrolysates from blueberry. One gram of ground blueberry was mixed with 100 mL of distilled water and then 100 µL (or 100 mg) of enzyme was mixed. Then, the relevant pH (using 1M HCl/1M NaOH) and temperature were adjusted to optimize the digestion process in each sample. Hydrolysis was performed for 12 h to reach the optimum degree of hydrolysis. Later, the samples were kept in a boiling water bath (100℃) for 10 min to inactivate the enzymes. Enzymatic hydrolysates were obtained after filtering via vacuum filtration and adjusted to pH 7.
Yields of the hydrolysates were calculated by dry weight of digested filtrate over dry weight of the blueberry used.
Total phenolic content was determined according to the method described by Chandler and Dodds [14] with slight modifications. One millilitre of blueberry hydrolysate was mixed in a tube containing 1 mL of 95% ethanol (v/v), 5 mL of distilled water and 0.5 mL of 50% Foiln-Ciocalteu reagent (v/v). The resultant mixture was allowed to react for 5 min and 1 mL of 5% Na2CO3 (w/v) was added. It was mixed thoroughly and placed in the dark for 1 h and absorbance was recorded at 725 nm wave length via UV-VIS spectrophotometer. A gallic acid standard curve was obtained for the calculation of phenolic content.
Chinese hamster lung fibroblast cell line (V79-4) was cultured in DMEM containing 10% fetal bovine serum, streptomycin (100 µg/mL) and penicillin (100 unit/mL) at 37℃ under a humidified atmosphere of 5% CO2 in air. Adherent cells were detached by scraping and then placed on a 96-well plate and used for experiment at 70-80% confluence. In a pilot investigation, cells were treated with H2O2 at concentrations ranging from 0.5 to 1 mM and then examined for cell viability. Accordingly, 1.0 mM H2O2 concentration was selected for this study.
The cell viability was estimated by MTT assay, which is a test of normal metabolic status of cells based on the assessment of mitochondrial activities [15]. V79-4 cells were seeded in a 96-well plate at a concentration of 1.0×105 cells/mL. After 16 h, the cells were treated with different concentrations of hydrolysates and incubated at 37℃ for 1 h. Then, 1 mM of H2O2 was added as final concentration and incubated for another 24 h at 37℃. Thereafter, a 50 µL of MTT stock solution (2 mg/mL) was added and incubated for 4 h. Then, the plate was centrifuged at 2,000 rpm for 5 min and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 mL of DMSO and absorbance was measured by ELISA reader (Tecan Co., Salzburg, Austria) at 540 nm wavelength. The optical density of the formazan formed in the control cells was taken as 100%.
The oxidation-sensitive dye DCFH-DA was used to determine the formation of intracellular ROS [16]. V79-4 cells were seeded in a 96-well plate at a concentration of 1.0×105 cells/mL. After 16 h, the cells were treated with different concentrations of hydrolysates and incubated at 37℃ for 30 min. Then, 1 mM H2O2 was added as final concentration and the cells were incubated for an additional 30 min at 37℃. After that, 5 mg/mL DCFH-DA was added to the cells and detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a Perking-Elmer LS-5B spectrofluorometer.
The lipid peroxidation was evaluated by measuring malondialdehyde by the method described by Ohkawa et al. [17]. V79-4 cells were seeded in a culture dish at a concentration of 1.0×105 cells/mL and incubated at 37℃. After 16 h, cells were treated with different concentrations of hydrolysates (50 and 100 mg/mL). One hour later, 1 mM of H2O2 was added and cells were incubated for an additional hour. The cells were then harvested and washed with PBS and homogenized in ice-cold 1.15% (w/v) KCl. A 100 µL of the cell lysate was combined with 0.2 mL of 8.1% (w/v) sodium dodecylsulfate (SDS), 1.5 mL of 20% (v/v) acetic acid (pH 3.5), and 1.5 mL of 0.8% thiobarbituric acid (w/v). The mixture was brought to a final volume of 4.0 mL with distilled water, and heated at 95℃ for 2 h. After cooling to room temperature, 5.0 mL of a mixture of n-butanol and phyridine (15:1 v/v) was added and mixed thoroughly. After centrifugation at 1,500 rpm for 10 min, the supernatant was separated and the absorbance was measured at 532 nm wave length.
First, V79-4 cells (4×104 cells/mL) were incubated without hydrolysates for 30 min at 37℃ (I). Second, V79-4 cells (4×104 cells/mL) were incubated without hydrolysates for 30 min at 37℃ but damaged oxidatively with 50 µM H2O2 for 5 min on ice (II). Third, V79-4 cells (4×104 cells/mL) were incubated with hydrolysates for 30 min at 37℃ and treated with 50 µM H2O2 for 5 min on ice (III). After each treatment, the cell suspension was centrifuged at 1500 rpm for 5 min, and washed with PBS.
Then, alkaline comet assay was conducted as described by Singh et al. [18] with slight modifications. The cell suspensions prepared in the previous steps were mixed with 100 µL of 0.7% (w/v) low melting agarose (LMA), and added to the slides precoated with 1.0% (w/v) normal melting agarose. After solidification of the agarose, slides were covered with another 100 µL of 0.7% (w/v) LMA, and then immersed in lysis buffer (2.5 M NaCl, 500 mM EDTA, 1 M Tris buffer, 1% (v/v) sodium laurylasarcosine, and 1% (v/v) Triton X-100) for 90 min. Later, the slides were transferred into an unwinding buffer for another 20 min for DNA unwinding. The slides were then placed in an electrophoresis tank containing 300 mM NaOH and 1 mM Na2EDTA (pH 13.0). For electrophoresis of the DNA, an electric current of 25 V/300 mA was applied for 20 min. After that the slides were washed two times with a neutralizing buffer (0.4 M Tris, pH 7.5) for 10 min, and treated with ethanol for another 5 min before staining with 40 µL of ethidium bromide (20 µL/mL). Measurements were made by image analysis (Komet, Andor Technology, Belfast, U.K) and fluorescence microscope (LEICA DMLB, Wetzar, Germany), determining the percentage of fluorescence in the tail (tail intensity, TI; 50 cells from each of two replicate slides).
The nuclear morphology of the cells was evaluated using a cell-permeable DNA dye, Hoechst 33342. The cells with homogeneously stained nuclei were considered viable, whereas the presence of chromatin condensation and/or fragmentation was the indication of apoptosis [19]. Vero cells were seeded in a 96-well plate at a concentration of 1.0×105 cells /mL. After 16 h, the cells were treated with the hydrolysates at 100 mg/mL concentration and incubated at 37℃. Two hours later, 1 mM H2O2 was added and cells were incubated at 37℃. After 24 h, 1.5 µL of (stock 10 mg/mL) Hoechst 33342 dye was added to each well and incubated at 37℃ for 10 min. Then, stained cells were observed under a fluorescence microscope equipped with a CoolSNAP-Pro colour digital camera.
The yield content due to hydrolysis by carbohydrases and proteases was more than 40% in each hydrolysate (Fig. 1). Total phenolic content (TPC) was ranged between 500.6 and 684.7 mg/100 g while Alcalase hydrolysate showed the highest TPC (684.7 mg/100 g).
The viability of the cells treated with various hydrolysates was compared with that of the cells treated only with vehicle control (NC: negative control) and all hydrolysates showed more than 90% cell viability at the tested concentration, revealing no significant difference (P < 0.05) of cytotoxicity between treatment and vehicle control (data not shown). The reduction of cell viability was measured after induction with 1 mM H2O2 by MTT assay and showed that the survival rate was reduced to 40.2% in the cells treated only with H2O2 for 24 h (Fig. 2). The pre-incubation of cells with hydrolysates showed more than 34% inhibitory effect against H2O2-induced cell damage at highest concentration. AMG (60.88%) and Alcalase (59.46%) hydrolysates showed the highest cell viability among carbohydrase and protease hydrolysates, respectively.
The scavenging effect of hydrolysates against intracellular ROS formation was studied using H2O2 as free radical generator. The DCF fluorescence was highly increased in the cells treated only with H2O2 showing significantly reduced (P < 0.05) ROS scavenging activity. However, cells pretreated with the hydrolysates decreased the fluorescence intensity in the cells in a dose-dependent pattern and showed increased ROS scavenging activity. AMG and Alcalase hydrolysates showed the highest ROS inhibitory activities compared to those of other hydrolysates tested (Fig. 3) and the data were presented as % of positive control. Considering the results obtained for cell viability and ROS formation inhibition, AMG and Alcalase hydrolysates were selected for further antioxidant assays.
Inhibitory effect of the hydrolysates against oxidative cell damage caused by H2O2 was evaluated by lipid peroxidation assay (Fig. 4). Incubation of V79-4 cells with H2O2 significantly increased (P < 0.05) the thiobarbituric acid reactive substances (TBARS) compared to that of the negative control. However, the cells pretreated with hydrolysates decreased the TBARS formation in a dose-dependent pattern. AMG and Alcalase hydrolysates showed 54.9% and 57% inhibitory activities at 100 µg/mL, respectively.
Comet assay was used to assess whether hydrolysates protect H2O2-induced DNA damage in V79-4 cells. AMG hydrolysate showed 73.64% inhibitory effect against cell damage while Alcalase hydrolysate showed 71.21% inhibitory effect at 100 µg/mL (Fig. 5). Furthermore, fluorescence intensity in the tail of the positive control was increased due to damage caused by H2O2 induction while decreased fluorescence intensity was observed in the cells pre-treated with hydrolysates. Photomicrographs of different DNA migration profiles obtained from V79-4 cells treated with different concentrations of AMG hydrolysates were shown in Fig. 6. Results showed significant increase in DNA strand breaks after treatment of H2O2 and were indicated by elongated tail in comet image in the cells treated only with H2O2 compared to that of the negative control cells. However, pretreatment of cells with AMG hydrolysates decreased the damage in a concentration-dependent manner and could be observed as a reduced tail. Therefore, H2O2-induced DNA damage was successfully overcome/repaired by the active compounds present in blueberry enzymatic hydrolysates.
To evaluate the morphological changes of H2O2-induced V79-4 cells, the nuclei were stained with Hoechst 33342 and observed by microscope. The microscopic pictures (Fig. 7A-D) showed that the control cells had intact nuclei, while the H2O2 treated cells showed significant nuclear fragmentation, which was an indication of apoptosis (indicated by arrows). However, when the cells were pre-treated with the hydrolysates for 2 h prior to H2O2 treatment, a decrease in nuclear fragmentation was observed.
Phenolic compounds that are known to possess high antioxidative activity are common phytochemicals in fruits and leafy vegetables. Previous studies have shown that there was a direct relationship between antioxidant activity and phenolic compounds in herbs, vegetables and fruits. The predominant flavonoids found in blueberry are anthocyanins and flavanols. Anthocynins can exist as diglycosides or acylated forms. Ellagitanins are also present in blueberry as a major pytochemical that can be metabolized into ellagic acid. In this study, we evaluated total phenolic content as gallic acid equivalents using Folin-Ciocalteu method. Those phytochemicals possess some beneficial health effects on oxidative damage, detoxification enzymes, and the immune system while they appear to have a synergistic effect on vitamin C and other flavonoids [20]. Polysaccharide and small protein molecules may also be included in the enzymatic hydrolysates revealing their activities due to synergetic effect of all those constituents including phenolic compounds. Many researchers have worked on anticancer activities of blueberry [21,22]. However, just a few studies have reported antioxidant activity [23]. Besides, no study about the enzymatic hydrolysis of blueberry has been reported so far. In this study, we are the first reporting intracellular antioxidant effect of the hydrolysates from blueberry with V79-4 cells.
Hydrogen peroxide is a representative ROS that is produced during the redox process and is believed to play a role as a messenger in intracellular signaling cascades [24]. Though, H2O2 is not a reactive molecule yet it forms highly reactive hydroxyl radicals by the Fenton reaction [25], and those radicals react rapidly with almost every cellular macromolecule including DNA, lipids and proteins by producing functional and structural alterations in those biomolecules.
Previous studies indicate that cells treated with H2O2 cause a marked decrease in cell survival, elevation of oxidative stress characterized by malondialdehyde (MDA) production and LDH release, and reduction in SOD, CAT and GSH-Px activities. Further, H2O2 exposure causes a rising Ca2+ concentration in the cytoplasm [26]. Different processes of degradation, such as ROS formation [27] and activation of several hydrolytic enzymes are initiated by elevated level of Ca2+ [28]. In addition, caspase-3 can be activated by H2O2 and disrupts the intracellular homeostasis of Ca2+ [29]. Therefore, the addition of H2O2 into the cells may lead critical DNA damage. However, antioxidant compounds have been shown potentiality to scavenge H2O2 and the structural requirements of the antioxidant compounds for efficient quenching of hydrogen peroxide are rather more complicated than those established for radical scavenging activities [30].
The cytotoxicity of hydrolysates from blueberry reveals that it possesses a clear concentration range in which toxic effects are not observed at the concentrations tested (data not shown). The results showed that pre-treatment with hydrolysates protected cells from the oxidative damage caused by H2O2 as observed in the increased cell survival. Accumulation of intracellular ROS can be detected by DCFH-DA, which is a permeable dye through cell membrane. The DCFH-DA is hydrolyzed to DCF by the esterase activity that can be trapped intracellularly. DCF is then able to interact with peroxides and forms the fluorescent 2', 7'-dichlorofluorescien, which is readily detected by spectroflurometer. In this study, it was found that H2O2-induced intracellular accumulation of ROS was attenuated by pretreatment with hydrolysates. In this study, hydrolysates from blueberry significantly decreased intracellular ROS generation, revealing that the protective effect is associated with the scavenging of intracellular ROS by the hydrolysates.
Antioxidants interrupt lipid (and protein) oxidation, either in the propagation phase (chain-breaking mechanism) or by protecting the oxidation substrates against the first radicals formed in the initiation phase. In the biological system, a number of degradation products such as malondialdehyde (MDA) are generated and found to be an important cause of cell membrane destruction and cell damage [31]. Hydrogen peroxide is potentially cytotoxic and generates hydroxyl radicals by the reaction between H2O2 and transition metal ions such as Fe2+ and Cu2+ in biological systems [32]. These hydroxyl radicals can react with a number of target molecules including proteins, membrane lipids and DNA. Moreover, oxidation of lipids induced by the hydroxyl radicals can generate end-products, such as MDA and unsaturated aldehydes, that can bind to DNA to generate mutagenic adducts [33]. From the result, it is thought that the hydrolysates from blueberry display a significant protective capability against H2O2-induced cell damage.
The comet assay has been described as a sensitive, rapid and inexpensive screening test for evaluating DNA damage caused by ROS [34]. DNA, which is the genetic material in the cells that controls cellular functions, can be damaged as a result of several factors such as ROS, smoke, heat, toxic chemicals and ultraviolet light. The sequence of the DNA base pair can be changed and led errors/disorders in replicating DNA if the damage cannot be repaired by the existing DNA repair mechanisms. The DNA damage of cultured V79-4 cells was induced by H2O2 and the ability of hydrolysates to inhibit the damage was extrapolated by the fluorescence intensity of the tail extent movement. Wilms et al. [35] reported that the intake of blueberry juice has a significant dose-dependent protection against oxidative DNA damage. Further, irreparable DNA damage is induced in carcinogenesis, aging and many other degenerative diseases [36]. Therefore, it is thought that the hydrolysates from blueberry which inhibit oxidative DNA damage can exert preventive action on cancer development.
ROS such as H2O2 and hydroxyl radicals readily damage biological molecules including DNA and protein which can eventually lead to apoptic or necrotic cell death [37]. Therefore, removal of excess ROS or suppression of their generation by antioxidants may be effective in preventing oxidative cell death. In this assay, only nuclear morphological changes were evaluated using Hoechst 33342 dye specifically staining DNA and observed through a fluorescence microscope. This method is widely used to detect nuclear shrinkage, chromatin condensation, nuclear fragmentation, and the appearance of apoptotic bodies which are considered as indications of apoptosis [38]. Hence, this method is known as a semi-quantitative method as the morphological changes are not quantified via any digital image analysis method in this assay. ROS initiate apoptosis by stimulating plasma membrane death receptors, but ROS, particularly H2O2 are also produced as a consequence of activation of these receptors by their ligands, and these latter ROS are also believed to participate in the apoptotic body formation process. Despite considerable progress in the understanding of the mechanistic basis of apoptosis, morphological analysis remains unquestionably the "gold standard" for its assessment and quantitation [39].
In conclusion, high amount of yield and water soluble phenolic compounds are resulted in the digestion process by food grade enzymes. The results from in vitro experiments demonstrated that the phytochemicals in enzymatic hydrolysates from blueberry have significant effect on H2O2-induced cell damage. Under the described experimental conditions, the hydrolysates from blueberry showed protective effect against cytotoxicity, lipid peroxidation, DNA damage, and apoptotic body formation induced by H2O2. Further studies are needed to isolate and characterize the active compounds available in blueberry hydrolysates.
Figures and Tables
Fig. 1
Total phenolic content and yield of hydrolysates obtained by treatment of different enzymes. Experiments were conducted in triplicate and data are expressed as means ± SD. Total phenolic content was evaluated via Folin-Ciocalteu method and expressed as the gallic acid equivalents.

Fig. 2
Cytoprotective effect of hydrolysates from blueberry on V79-4 cells. V79-4 cells were incubated (1×105 cells/mL) for 16 h and induced with 1 mM H2O2 for 24 h with or without various hydrolysates. Cell viability was evaluated by MTT assay. Hydrolysates were tested at the concentrations of ▒50 µg/mL and □100 µg/mL in triplicate. Data were expressed as means ± SD. NC: negative control, PC: positive control.
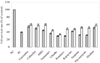
Fig. 3
ROS scavenging activity of enzymatic hydrolysates from blueberry in V79-4 cells. V79-4 cells were incubated (1×105 cells/mL) for 16 h and induced with 1 mM H2O2 for 30 min with or without various hydrolysates. The oxidation-sensitive dye DCFH-DA was used to determine the formation of intracellular ROS. Data were presented as % of positive control. Hydrolysates were tested at the concentrations of ▒50 µg/mL and □100 µg/mL in triplicate. Data were expressed as means ± SD. NC: negative control.
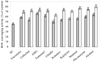
Fig. 4
Lipid peroxidation inhibitory effect of Alcalase and AMG hydrolysates from blueberry. V79-4 cells were incubated (1×105 cells/mL) for 16 h and induced with 1 mM H2O2 for 1 h with or without various hydrolysates. Lipid peroxidation products were evaluated by TBA. Bars filled in gray color: Alcalase hydrolysate and bars without filling: AMG hydrolysate; lipid peroxidation inhibitory effect of Alcalase: -▴- and AMG: -x-. *P < 0.05, significantly different from H2O2 alone. NC: negative control. 0 indicates and vehicle control (data not shown). The reduction of cell positive control

Fig. 5
Effect of Alcalase and AMG hydrolysates on DNA damage induced by H2O2 in V79-4 cells. V79-4 cells were incubated (4×104 cells/mL) for 16 h and pre-treated with or without various hydrolysates for 30 min. Then, induced with 50 µM H2O2 for 5 min and DNA damage was determined by comet assay. Bars filled in gray color: Alcalase and bars without filling: AMG; Inhibitory effect of cell damage of Alcalase hydrolysate: -▴- and AMG hydrolysate: -x-. *P < 0.05, significantly different from H2O2 alone. NC: negative control, 0 indicates positive control

Fig. 6
Comet images of V79-4 cells obtained for AMG hydrolysate. Measurements were made by image analysis with fluorescence microscope determining the percentage of fluorescence in the tail. (A) negative control (B) V79-4 cells treated with 50 µM H2O2 (Positive control) (C) V79-4 cells treated with 50 µg/mL AMG hydrolysate+50 µM H2O2 (D) V79-4 cells treated with 100 µg/mL AMG hydrolysate+50 µM H2O2
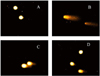
Fig. 7
Effect of hydrolysates from blueberry on apoptosis. The nuclear morphology of the cells was evaluated using a cell-permeable DNA dye, Hoechst 33342. A: Negative control, B: H2O2 treated sample (positive control), C: 1 mM H2O2+100 µg/mL of Alcalase hydrolysate, D: 1 mM H2O2+100 µg/mL of AMG hydrolysate

References
1. LaFerla FM. Calcium dyshomeostasis and intracellular signaling in Alzheimer's disease. Nat Rev Neurosci. 2002. 11:862–872.
2. Prasad KN, Cole WC, Hovland AR, Prasad KC, Nahreini P, Kumar B, Edwards-Prasad J, Andreatta CP. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr Opin Neurol. 1999. 12:761–770.

3. Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001. 64:43–51.

4. Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2001. 29:323–333.

5. Triggiani V, Resta F, Guastamacchia E, Sabba C, Licchelli B, Ghiyasaldin S, Tafaro E. Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and trace elements in the treatment of diabetes mellitus and its chronic complications. Endocr Metab Immune Disord Drug Targets. 2006. 6:77–93.

6. De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005. 591:8–15.

7. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39:44–84.

8. Prior RL, Cao G, Martin A, Sofic E, McEwen J, O'Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. 1998. 46:2686–2693.

9. Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nut Food Res. 2007. 51:652–664.

10. Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova N, Bickford PC, Joseph JA. Short-term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000. 3:383–397.

11. Gruenwald J. PDR for Herbal Medicines. 2004. Newjersy: Thomson PDR publisher;228–235.
12. Chambers B, Camire M. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care. 2003. 26:2695–2696.

13. Heo SJ, Lee KW, Song CB, Jeon YJ. Antioxidant activity of enzymatic extracts from brown seaweeds. Algae. 2003. 18:71–81.

14. Chandler SF, Dodds JH. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasidine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1993. 2:105–110.
15. Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989. 119:203–210.

16. Engelmann J, Volk J, Leyhausen G, Geurtsen W. ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res Part B Appl Biomater. 2005. 75:272–276.

17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.

18. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988. 175:184–191.

19. Gschwind M, Huber G. Apoptotic cell death induced by β-amyloid 1-42 peptide is cell type dependent. J Neurochem. 1995. 65:292–300.

20. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005. 16:77–84.

21. Schmidt BM, Howell AB, Mceniry B, Knight CT, Seigler D, Erdman JW Jr, Lila MA. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. J Agric Food Chem. 2004. 52:6433–6442.

22. Yi W, Akoh CC, Fischer J, Krewer G. Effects of phenolic compounds in blueberries and muscadine grapes on HepG2 cell viability and apoptosis. Food Res Int. 2006. 39:628–638.

23. Sellappan S, Akoh CC, Krewer G. Phenolic compounds and antioxidant capacity of Georgia-Grown blueberries and blackberries. J Agric Food Chem. 2002. 50:2432–2438.

24. Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999. 31:53–59.

25. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 1989. Oxford: Oxford University Press;96–98.
26. Wang H, Joseph JA. Mechanisms of hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med. 2000. 28:1222–1231.

27. Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998. 141:1423–1432.

28. Castillo MR, Babson JR. Ca2+-dependent mechanisms of cell injury in cultured cortical neurons. Neuroscience. 1998. 86:1133–1144.

29. McGinnis KM, Wang KKW, Gnegy ME. Alterations of extracellular calcium elicit selective modes of cell death and protease activation in SH-SY5Y human neuroblastoma cells. J Neurochem. 1999. 72:1853–1863.

30. Mansouri A, Makris DP, Kefalas P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescence-based assay: Structure-activity relationships. J Pharm Biomed Anal. 2005. 39:22–26.

31. Yoshikawa T, Naito Y, Kondo M. Hiramatsu M, Yoshikawa T, Inoue M, editors. Food and disease. Free radicals and diseases. 1997. New York: Plenum Press;11–19.
32. Halliwell B, Gutteridge MC. Oxygen toxicity, oxygen radical, transition metals and disease. Biochem J. 1984. 219:1–14.
33. Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994. 265:1580–1582.

34. Kassie F, Parzefall W, Knasmuller S. Single cell gel electrophoresis assay: a new technique for human biomonitoring studies. Mutat Res. 2000. 463:13–31.

35. Wilms LC, Hollman PCH, Boots AW, Kleinjans JCS. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat Res. 2005. 582:155–162.

36. Cozzi R, Ricordy R, Aglitti T, Gatta V, Perticone P, De Salvia R. Ascorbic acid and beta-carotene as modulators of oxidative damage. Carcinogenesis. 1997. 18:223–228.

37. Jang JH, Surh YJ. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res. 2001. 496:181–190.

38. Kerr JF, Gobe GC, Winterford CM, Harmon BV. Schwartz LM, Osborne BA, editors. Anatomical methods in cell death. Methods in cell biology. 1995. New York: Academic Press;1–27.
39. Vicki S, Peter AH, Philip JC. Brady JM, editor. Detecting and quantifying apoptosis in tissue sections. Apoptosis methods and protocols. 2004. New Jersey: Humana Press;67–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download