Abstract
In our previous study, we have shown that berberine has both anti-adipogenic and anti-inflammatory effects on 3T3-L1 adipocytes, and the anti-adipogenic effect is due to the down-regulation of adipogenic enzymes and transcription factors. Here we focused more on anti-inflammatory effect of berberine using real time RT-PCR and found it changes expressions of adipokines. We hypothesized that anti-adipogenicity of berberine mediates anti-inflammtory effect and explored leptin as a candidate mediator of this signaling. We studied this hypothesis by western blot analysis, but our results showed that berberine has no effect on the phosphorylations of STAT-3 and ERK which have important roles on leptin signaling. These results led us to conclude that the anti-inflammatory effect of berberine is not mediated by the inhibition of leptin signal transduction. Moreover, we have found that berberine down-regulates NF-κB signaling, one of the inflammation-related signaling pathway, through western blot analysis. Taken together, the anti-inflammatory effect of berberine is not mediated by leptin, and berberine induces anti-inflammatory effect independent of leptin signaling.
Berberine is an isoquinoline derivative alkaloid isolated from many medicinal herbs, such as Hydrastis Canadensis (goldenseal), Cortex phellodendri (Huangbai) and Rhizoma coptidis (Huanglian) (Ikram, 1975). Several studies have shown that berberine has many pharmacological effects including inhibition of adipocyte differentiation, LDL-lowering effects and anti-inflammatory potential (Choi et al., 2006; Kong, 2004; Kuo et al., 2004). However, the anti-adipogenic and anti-inflammatory mechanism of berberine has not been elucidated clearly yet.
Recently, the evidence from many studies indicates that obesity is closely related with inflammation (Fantuzzi, 2005). Adipocytes actually secrete many inflammatory molecules such as leptin, TNF-α, PGE2, adiponectin, etc. (Hotamisligil et al., 1993; Trayhurn & Wood, 2004). Especially, the role of leptin in inflammation has become increasingly evident and has been reviewed recently (La Cava & Matarese, 2004). Not only leptin has structural and functional similarity to other pro-inflammatory cytokines such as TNF-α and IL-6, but also its signaling is accelerated by pro-inflammatory cytokines (Baumann et al., 1996; Otero et al., 2006). The binding of leptin to leptin receptor results in the activation of JAK-2 and leads to the phosphorylation of cytoplasmic target proteins, including STAT-3 and the MAPK (Lam & Lu, 2007). As with leptin, adiponectin is produced mainly by adipocytes. However, in contrast to leptin, adiponectin is considered to be an anti-inflammatory cytokine and has anti-inflammatory effects in various animal models of liver inflammation (Bastard et al., 2006).
Some research groups are making their efforts on elucidating the anti-adipogenic and anti-inflammatory effects of berberine (Enk et al., 2007; Hu et al., 2008; Kim et al., 2008; Kuo et al., 2004), although how berberine makes these two effects is not elucidated yet. Previously, we reported that berberine has an anti-adipogenic effect by reducing the expression of adipogenic transcription factors and the mRNA expressions of inflammatory molecules (Choi et al., 2006). This result led us to hypothesize that leptin, which is dominantly synthesized in adipocytes and related with inflammation, is the molecule that mediates the anti-inflammatory effects of berberine in adipocytes. In other words, berberine reduces fat accumulation and further down-regulates leptin synthesis, so the decrease of leptin signaling in an autocrine way might be in charge of the reduction of inflammatory molecule expressions.
Therefore, in this study, we examined the effect of berberine in mRNA expressions of adipokines by real-time RT-PCR. Then we investigated whether the reduced expressions of inflammatory molecules by berberine are due to the down-regulation of leptin signaling. For this purpose, we examined the change of leptin signaling especially STAT-3 and ERK phosphorylations by berberine, by western blot analysis. Furthermore, we examined I-κB phosphorylation in order to investigate NF-κB signaling.
High-glucose DMEM, BCS, and FBS were purchased from Hyclon (Logan, Utah, USA). Trypsin/EDTA, insulin, dexamethasone, IBMX, and MTT which is used in cell culture and sample treatment were purchased from Sigma (St. Louis, MO, USA).
Trizol for RNA extraction was purchased from Invitrogen (Carlsbad, CA, USA), Superscript II reverse transcriptase was purchased from BD Bioscience (San Jose, CA, USA), 2 X SYBR green Mix was purchased from ABI Applied Bioscience (Foster City, CA, USA) and all primers and oligo dT primer were purchased from Bioneer (Daejeon, Korea).
Antibody against ERK1/2, phosphor-ERK1/2, β-actin, anti-rabbit IgG-horseradish peroxidase-conjugated, anti-mouse IgG-horseradish peroxidase-conjugated were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-STAT3 and anti-phospho-STAT3 antibodies were purchased from Biosource (Camarillo, CA, USA) and anti-IκB and anti-phospho-IκB were from Cell Signaling (Danvers, MA, USA).
Mouse 3T3-L1 pre-adipocytes were grown to confluence in DMEM with 10% BCS at 37℃ in a humidified atmosphere of 5% CO2. At 1 day post-confluence (designated "day 0"), cell differentiation was induced with a mixture of methylisobutylxanthine (0.5 mM), dexamethasone (0.25 mM), and insulin (5 mg/ml) in DMEM containing 10% FBS. On day 2 and thereafter, DMEM containing 10% FBS and insulin (5 mg/ml) only was subsequently replaced every 2 days.
Berberine was dissolved in DMSO, divided into small quantities, and stored in -20℃. At day 8, after inducing differentiation of adipocytes, the sample was dissolved in DMEM at 10 mM concentration, which was treated into adipocytes for 4 hours. The final concentration of DMSO was less than 0.1%. After treating the samples for 4 hours, cells were used for RNA extraction, as described below.
Total RNA was isolated from 3T3-L1 adipocytes using Trizol reagent. First strand cDNA synthesis was performed with 2 mg of total RNA using Superscript II reverse transcriptase (BD bioscience, Palo Alto, CA, USA).
Real-time RT-PCR analysis was performed by Applied Biosystems 7500 Real Time PCR system (Applied Biosystems, Foster City, CA, USA). Samples (mixture that 2 X SYBR green Mix, 0.5 µM each appropriate primers, and cDNA) were incubated in the Applied Biosystems 7500 Real Time PCR system for an initial denaturation at 94℃ for 10 min, followed by 40 PCR cycles. Each cycle consisted of 95℃ for 15s, 60℃ for 1minute. To confirm amplification of specific transcripts, melting curve profiles (cooling the sample to 65℃ for 15s and heating slowly to 95℃ with continuous measurement of fluorescence) were produced at the end of each PCR. The oligonucleotide primers for the experiments are: beta-actin; 5'-AGCCATGTACGTAGCCATCC-3' and 5'-TCCCTCTCAGCTGTGGTGAA-3', leptin; 5'-AGGATCTGAGGGGTGATGTGA-3' and 5'-TGAGGTGACCAAGGTGGCATAG-3', MCP-1; 5'-TTAAAAACCTGGATC GGAACCAA-3' and 5'-GCATTAGCTTCAGATTTACGGGT-3', adiponectin; 5'-GTC TCAGCTGTCGGTCTTCCCCT-3' and 5'-CCCTGGCTTTATGCTCTT TGC-3'.
After experimental treatment, cells were washed twice with ice-cold phosphate-buffered saline and lysed with extraction buffer (containing 50 mM Tris-HCl, pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM EDTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 100 mM NaF, 1 mM PMSF and protease inhibitor cocktail on ice for 30 min. Insoluble materials were removed by centrifugation at 15,000g for 10 min at 4℃. The total concentration of extracted proteins was determined using the method of Bradford. The proteins in the supernatants were separated by 10% SDS-PAGE and transferred into nitrocellulose membranes. After blocking with TBS-T (10 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 7.6) containing 5% nonfat milk for 1 h at room temperature, the membranes were incubated with the appropriate first antibodies. To detect the antigen-bound antibodies, the blots were treated with secondary antibodies conjugated with horseradish-peroxidase. Immuno-reactivity was detected by using the ECL Western blotting detection system.
Previously, we showed that the mRNA expressions of inflammatory molecules such as TNF-α, IL-6, CRP, and Haptoglobin are decreased by berberine treatment in 3T3-L1 adipocytes. Here we focused more on adipokines and studied how their mRNA expressions are influenced by berberine treatment. We treated berberine for 18 hrs on fully differentiated adipocytes and examined mRNA expression levels of leptin, adiponectin, and MCP-1. Leptin and MCP-1 mRNA expressions were greatly decreased about 80-90%, but adiponectin which has a special role in anti-inflammation was increased by 35% after treatment of berberine (Fig. 1-a). In order to investigate when the inflammatory molecules are reduced mostly after a treatment of berberine, we examined the time course of TNF-α and leptin mRNA expressions in berberine-treated 3T3-L1 adipocytes. The mRNA expressions of TNF-α and leptin were estimated by real time RT-PCR. The mRNA expression of TNF-α was decreased mostly 4 hours later, then increased by 6 hours of treatment and went back to the original level of expression at 24 hours of treatment (Fig. 1-b). The mRNA expression of leptin was decreased more than 40% during all the times of berberine treatment (Fig. 1-c). Therefore, we chose 4 hours berberine-treated samples for the further investigations.
Anti-adipogenic and anti-inflammatory effects of berberine were shown in Fig. 1 and our previous report (Choi et al., 2006). Through these data, we assumed that reduced adipogenisity by berberine induces down-regulation of inflammatory molecules. We chose the leptin signaling as the best candidate to explore and have examined the effect of berberine on STAT-3 phosphorylation and ERK phosphorylation. STAT-3 and ERK phosphorylations were measured by western blotting and STAT-3 and ERK were used as internal control, respectively. However, contrary to our expectation, the phosphorylations of STAT-3 and ERK were not significantly changed after berberine treatment. (Fig. 2-a, b).
According to the previous experiments, berberine does not seem to inhibit the leptin signaling pathway. Therefore, we investigated the anti-inflammatory effect of berberine itself instead of examining berberine's anti-inflammatory effect in accordance with its anti-adipogenic effect. In this regard, we have investigated the activity of NF-κB which is the representative transcription factor of inflammation. Inactive NF-κB exists as a dimer with I-κB and along with the I-κB phosphorylation, NF-κB becomes activated (Ghosh et al., 1998). So we have examined the changes in I-κB phosphorylation by berberine in 3T3-L1 adipocytes. And we have found approximately a 57% decrease of I-κB phosphorylation (Fig. 3).
In this study, we have shown that berberine reduces the expression of leading adipokines such as leptin and MCP-1 and increases adiponectin expression. Berberine mostly reduces TNF-α and leptin expression levels at 4 hours of treatment. However, berberine does not have an effect on phosphorylation of STAT-3 or ERK, key proteins of leptin signaling. Berberine has a reducing effect on I-κB phophorylation which is in charge of NF-κB activation.
In our previous report, we have shown that berberine reduces the expression levels of 3T3-L1 adipocyte-secreted inflammatory molecules (Choi et al., 2006). Here we focused more on key adipokines and found berberine has an anti-inflammation effect on adipocytes. Then we were intrigued to know about the most effective time of berberine treatment and examined the expression of TNF-α and leptin in a time-dependent manner. TNF-α expression was the lowest and leptin expression was mostly down-regulated on two to four hours of berberine treatment. Through this, our group hypothesized that the anti-inflammatory effect of berberine might be mediated by leptin. In other words, the decrease of adipocyte differentiation by berberine treatment results in the decrease of leptin secretion, so that leptin-induced inflammation is diminished and finally the expressions of inflammatory molecules are declined.
In this regard, we examined that the phosphorylation of STAT-3 which has a critical role in leptin receptor signaling. To our surprise, the phosphorylation level of STAT-3 in the berberine-treated sample was almost the same as the control. We also explored on activation of ERK by berberine, another key pathway in leptin signaling. However, ERK phosphorylation was not affected by berberine treatment as well. In addition, the levels of STAT-3 phosphorylation and ERK phosphorylation were not changed by leptin treatment as well. According to Machinal-Quélin, leptin has proadipogenic effect on preadipocytes by activating STAT-3 and MAPK signaling pathways but on differentiated adipocytes, leptin has no effect on both signaling pathways (Machinal-Quélin et al., 2002).
From these results, we concluded that berberine does not inhibit leptin signaling. So we examined NF-κB signaling pathway as another candidate pathway for anti-inflammatory effect of berberine and we found berberine decreases I-κB phosphorylation. Recently, studies on berberine have reported that the anti-inflammatory effect of berberine is induced by reducing NF-κB signaling pathway in adipocytes, and one of them clarified that berberine suppresses IL-1β and TNF-α production through the inhibition of I-κB degradation and that result in the inhibition of NF-κB translocation (Hu et al., 2008; Lee et al., 2007). If the anti-inflammatory effect of berberine is not mediated by leptin, NF-κB could be another molecule which is responsible for that. Therefore, NF-κB signal transduction seems to be responsible for anti-inflammatory effect of berberine.
Until now, we have investigated the anti-inflammatory effect of berberine and its underlying mechanism. We primarily considered that berberine has anti-inflammatory effect in accordance with its anti-adipogenic effect. So we hypothesized that leptin is the molecule that relates two effects of berberine on 3T3-L1 adipocytes. However, leptin signaling has not been affected by berberine treatment but NF-κB signaling has been changed instead. In conclusion, the anti-inflammatory effect of berberine is not mediated by anti-adipogenicity but by a different mechanism possibly NF-κB pathway.
Figures and Tables
 | Fig. 1
mRNA expressions of adipokines in BBR-treated 3T3-L1 adipocytes. At day 7 after inducing differentiation, adipocytes were cultured in 6-well plates with or without BBR for 18 hours. (a) Graph reporesents mRNA expressions of adipokines such as leptin, MCP-1, and adiponectin. Time course treatment berberine was conducted for 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours. Graphs represent mRNA expressions of (b) TNF-α and (c) leptin. (*p<0.05, **p<0.01, ***p<0.001) Data are expressed relative to untreated control cells and represent means ± S.E. Experiments were conducted twice, each included triplication. |
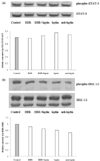 | Fig. 2
Leptin signaling in BBR-treated 3T3-L1 adipocytes. At day 8 after inducing differentiation, adipocytes were cultured in 6-well plates with or without BBR for 4 hours. Graphs represent (a) phosphorylation of STAT-3 and (b) phosphorylation of ERK. Experiments were conducted twice, each included triplication. |
References
1. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006. 17:4–12.
2. Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996. 93:8374–8378.

3. Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006. 38:599–605.

4. Enk R, Ehehalt R, Graham JE, Bierhaus A, Remppis A, Greten HJ. Differential effect of Rhizoma coptidis and its main alkaloid compound berberine on TNF-alpha induced NFkappaB translocation in human keratinocytes. J Ethnopharmacol. 2007. 109:170–175.

5. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005. 115:911–919.

6. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.

7. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993. 259:87–91.

8. Hu JP, Nishishita K, Sakai E, Yoshida H, Kato Y, Tsukuba T, Okamoto K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur J Pharmacol. 2008. 580:70–79.

9. Ikram M. A review on the chemical and pharmacological aspects of genus Berberis. Planta Med. 1975. 28:353–358.

10. Kim S, Kim Y, Kim JE, Cho KH, Chung JH. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine. 2008. 15:340–347.

11. Kong W. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004. 10:1344–1351.

12. Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004. 203:127–137.
14. Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007. 4:1–13.
15. Lee CH, Chen JC, Hsiang CY, Wu SL, Wu HC, Ho TY. Berberine suppresses inflammatory agents-induced interleukin-1beta and tumor necrosis factor-alpha productions via the inhibition of IkappaB degradation in human lung cells. Pharmacol Res. 2007. 56:193–201.

16. Machinal-Quélin F, Dieudonné MN, Leneveu MC, Pecquery R, Giudicelli Y. Proadipogenic effect of leptin on rat adipocytes in vitro: activation of MAPK and STAT3 signaling pathway. Am J Physiol Cell Physiol. 2002. 282:C853–C863.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download