Abstract
We investigated the nutritional state of B vitamins and the neuropsychological functions in 25 subjects, aged 63.1 ± 6.3 years, residing in rural areas of Korea. Nutritional states of thiamin, riboflavin, and pyridoxine were assessed enzymatically in the erythrocytes, and folate concentrations were measured microbiologically in the plasma and erythrocytes. A battery of composite neuropsychological test was administered to the subjects. Plasma folate was correlated with the total intelligence score (p=0.049). Folate levels in the erythrocytes were correlated with the performance intelligence scores such as block design (p=0.017) and picture arrangement (p=0.016). The red cell folate was correlated with memory scores such as general memory (p=0.009) and delayed recall (p=0.000). Although it did not reach statistical significance, verbal memory (p=0.053) was highly correlated with the red cell folate. The red cell folate was also correlated positively with the percent of conceptual level response number score (p=0.029), and negatively with the grooved pegboard test score for the non-dominant hand (p=0.010). Fine motor coordination was also influenced by folate nutrition, as finger tapping scores in both hands were significantly correlated with red cell folate (dominant hand; p=0.026, non-dominant hand; p=0.004). Other B vitamins such as thiamin, riboflavin, and vitamin B6 were not as strongly correlated with neuropsychological function test scores as folate was. These results suggest that folate nutrition influences neuropsychological function test scores significantly in humans. Further studies are needed to explore the relationship between folate or other vitamin B nutrition and neuropsychological functions and the implications thereof.
B vitamins such as thiamin, riboflavin, vitamin B6, and folate are known to be essential for the maintenance of normal metabolic functions in the brain. Thiamin and riboflavin coenzymes, thiamin pyrophosphate and flavin adenine dinucleotide and flavin mononucleotide namely, are involved in the mitochondrial energy metabolism in neurons (Baker & Tarnopolsky, 2003; Sheu et al., 1998). Vitamin B6 coenzyme, pyridoxal-5-phosphate is important for the metabolism of amino acids and proteins, neurotransmitters, and sphingolipids in the central nervous system (Dakshinamurti et al., 1985). Folate is required in the synthesis of S-adenosylmethionine (SAMe), a methyl donor for many important brain biomolecules such as phospholipids, guanidoacetate, neurotransmitters, amino acids, and nucleic acids (Bottiglieri et al., 2000; Hirata & Axelrod, 1980; Wagner, 1995).
Among other B vitamins, folate has received much attention recently as its low serum level is found to be closely associated with structural and functional abnormalities in the brain. Low serum folate levels have been related to atrophy of the cerebral cortex (Snowdon, 2000), dementia (Ramos et al., 2005), cerebrovascular diseases (Maxwell et al., 2002), and to specific domains of cognitive functioning such as episodic recall and recognition (Hassing et al., 1999; Wahlin et al., 1996). In addition to this, folate supplementation has shown a positive effect on cognitive functions (Durga et al., 2007; Nilsson et al., 2001) and memory deficits (Tettamanti et al., 2006).
Nutritional inadequacy for B vitamins is a frequently observed phenomenon among the elderly in developing countries (Fakhrzadeh et al., 2006) and also among those with low socioeconomic status in developed countries before a food folic acid fortification policy was mandated (Riggs et al., 1996) or in countries where folic acid fortification is not mandated (Chen et al., 2005; Joosten et al., 1993; Lim & Heo, 2002). The objective of the present study was to investigate the relations between vitamin B status in the blood and neuropsychological functions in free-living elderly subjects residing in rural areas of Korea.
Twenty-five elderly volunteers, 19 men and 6 women, residing on their own in rural areas of South Korea participated in the study. Participants with a history of medical, neurological or psychiatric illnesses, recent viral infection, use of antibiotics, or chronic metabolic diseases such as diabetes mellitus were excluded from the study. An informed consent form was obtained from each subject. The study was approved by the Human Investigation Review Committee of the Asan Medical Center.
Overnight fasting venous blood samples were collected in heparinized tubes and centrifuged at 1,600xg. After removing the plasma and buffy coat by aspiration, the erythrocytes were washed three times with ice-cold saline (0.9%). The packed erythrocytes were dispensed into 0.5 ml aliquots and were stored frozen at -70℃ until biochemical analysis was performed. The B vitamin status of thiamin, riboflavin, and vitamin B6 was assessed by conducting enzyme-coenzyme saturation kinetic assays on erythrocyte transketolase (TK; EC 2.2.1.1), glutathione reductase (GR; EC 1.6.4.2), and aspartate aminotransferase (AST; EC 2.6.1.1) (Bayoumi & Rosalki, 1976), respectively. Percent enzyme activations were calculated using the following equation.
(Enzyme activity with coenzymes-Enzyme activity without coenzymes)/ Enzyme activity without coenzymes×100
For blood folate status, folate concentrations in the plasma and red blood cells were determined microbiologically using Lactobacillus casei (ATCC 7469) (Buehring et al., 1974).
The participants underwent a battery of composite neuropsychological tests conducted by a neuropsychologist who was blinded to the subjects. Intelligence, memory, attention, problem solving abilities, and fine motor coordination were assessed using the following tests: the Korean standardized version (KWIS) of the revised version of the Wechsler Adult Intelligence Scale (WIS) for intelligence (Wechsler, 1981); the Wechsler Memory Scale, revised version (WMS-R) for memory (Wechsler, 1987); the Color Trail-making Test (CTT) for attention (Maj, 1993); the Wisconsin Card Sorting Test (WCST) for problem solving abilities (Heaton, 1981); and the Grooved Pegboard Test (GP) and Finger Tapping Test (FTT) for fine motor coordination (Ruff & Parker, 1993), respectively. We used an abbreviated version of the KWIS, using arithmetic and vocabulary sub-scale for the verbal intelligence, and block design and picture arrangement for performance intelligence. The neuropsychological test was performed on the same day as blood sample collection.
Test scores were found to be significantly influenced by age and years of education. The non-parametric Spearman's correlation analysis was performed between the neuropsychological test scores and blood B vitamin status with age and years of education as covariates. All statistical analyses were performed using a SPSS Statistical Analysis System Version 12.0 (Chicago, USA). Probability values less than 0.05 were considered statistically significant.
The characteristics of the study population are summarized in Table 1. The mean age of the respondents was 63.1 years and the mean years of education were 5.3 years. The anthropometric characteristics such as height, body weight and BMIs of these participants were similar to those of average rural people in Korea of 60-69 years of age.
In Table 2 are the percent activation of TK, GR, and AST, and the blood folate concentrations of the study subjects. The mean percent activation of TK, GR, and AST enzymes were 36.5 ± 24.6%, 43.1 ± 20.2%, and 98.8 ± 37.4%, respectively, which were higher than the values considered nutritionally adequate, thus indicating a suboptimal functional B vitamin status on average in these subjects. Mean folate concentrations in the erythrocytes and plasma were 406.6 ± 162.3 nmol/L and 21.3 ± 12.8 nmol/L, respectively, which indicated that on average the folate status of these subjects was considered nutritionally adequate.
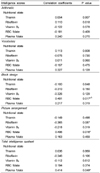
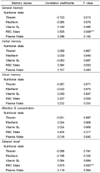
The adjusted correlation coefficients between blood B vitamin concentrations and cognitive test scores showed that red cell folate concentration was significantly correlated with the visuospatial abilities as shown in the positive correlation between red cell folate and the scores for block design (p=0.017) and picture arrangement (p=0.016). Plasma folate was significantly correlated with the total intelligence quotient scores (p=0.049) (Table 3). With regard to the correlation between blood B vitamin concentrations and memory scores, red cell folate was positively correlated with general memory (p=0.009) and delayed recall (p=0.000) scores. Although it did not reach statistical significance, verbal memory (p=0.053) was also highly correlated with the red cell folate (Table 4).
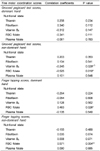
Table 5 shows the data on the relationship between B vitamin status and fine motor coordination scores. Red cell folate was negatively correlated with Grooved Pegboard Test scores of the non-dominant hand (p=0.010). Fine motor coordination was associated with folate status as finger tapping scores of both hands were significantly correlated (dominant hand; p=0.026, non-dominant hand; p=0.004) with red cell folate. In the Wisconsin Card Sorting Test, red cell folate was positively correlated with the percent of the conceptual level response number scores (p=0.029).
In our study, among other B vitamins, folate status as assessed by folate concentrations in the plasma and red blood cells was significantly correlated with test scores for several domains of cognitive function. Plasma folate was significantly correlated with total intelligence quotient scores, while red cell folate was correlated with the scores for memory, fine motor coordination, and problem solving ability.
Folate, along with vitamin B12, is known to be important for cognitive function. In elderly subjects, folate has been positively correlated with the auditory verbal learning test, digit symbol test, block design test, delayed recall, percentage of forgetting, and categorical verbal fluency test (Duthie et al., 2002; Feng et al., 2006). Elderly subjects with low serum folate have impaired spatial copying skills (Riggs et al., 1996) and abstraction performance (LaRue et al., 1997), and score lower on tests of nonverbal abstract thinking when compared with age-matched individuals with high serum folate concentrations (Goodwin et al., 1983).
The status of vitamin B6, vitamin B12 and folate is frequently inadequate in the elderly and several studies have shown associations between loss of cognitive function (Ramos et al., 2005; Smith, 2002; Tettamanti et al., 2006) or Alzheimer's disease (Snowdon et al., 2000) and inadequate B vitamin status. It is interesting, though, that in our study the proportion of subjects who were folate deficient was much lower when assessed by plasma (9.4%) and erythrocyte (19.8%) folate, respectively, than those (50-70%) for other B vitamins, and yet folate was the only vitamin which was related with cognitive function. An earlier study (Lindeman et al., 2000) found that cognitive function was significantly associated with serum folate concentrations, but not with vitamin B12, vitamin C and other vitamin concentrations in the serum of elderly subjects. In their study, the most significant associations observed were those between serum folate and various measures of cognitive function, even after adjusting for the presence of depression. Even in healthy older adults in developed countries with normal blood levels of B vitamins, test scores for delayed recall, abstract reasoning, and selective attention were shown to be positively associated with B vitamin levels in the serum (Bell et al., 1990).
Folate is found in high concentrations in the central nervous system. Some studies have found an association between folate deficiency and a variety of neurological and psychiatric disturbances such as impaired cognitive function, dementia and depression (Hassing et al., 1999; Ramos et al., 2005; Snowdon, 2000). Folate is important in one-carbon metabolism, contributing carbon atoms to purines, thymidine, and amino acids. Folate deficiency, by hindering the synthesis of DNA and protein synthesis for neuronal and glial growth and proliferation during critical points, can detrimentally influence neurotransmission and memory functions (Albright et al., 1999). Folate is also involved in maintaining adequate methionine pools for the synthesis of S-adenosylmethionine (SAMe) (Balaghi et al., 1993). SAMe is required in numerous transmethylation reactions involving nucleic acids, proteins, phospholipids, amines and other neurotransmitters (Bottiglieri et al., 1994). Folate deficiency can reduce SAMe concentrations in the central nervous system, thus increasing uracil misincorporation (Blount et al., 1997) and negatively affecting methylation of cellular DNA (Scott et al., 1994), proteins, and neurotransmitters, thus impeding neural function. Folate deficient mice also display behavioral abnormalities (Gospe et al., 1995). In the SENECA study, education and high plasma levels of folate and beta-carotene appear to be associated with a lower risk of developing dementia (Haller et al., 1996). Low concentrations of folate were associated with neocortical atrophy at autopsy in subjects with Alzheimer's disease (Snowdon et al., 2000).
In our study, folate concentrations in red cells had stronger associations with cognitive function than those in plasma, confirming the observation by Durga et al. (2007), who reported that low concentrations of erythrocyte folate but not serum folate were associated with poor cognitive performance. Since folate concentration in tissue reflects long-term body folate status better than that in serum (Varela-Moreiras & Selhub, 1992), we may have observed in our subjects a stronger association between cognitive function and red cell folate. In our subjects, the positive correlation of plasma folate was limited to total intelligence quotient scores whereas that of erythrocyte folate was with other domains of cognitive function such as memory, fine motor coordination and Wisconsin Card Sorting Test scores. If we had a larger number of subjects in the study, we might have been able to see positive associations between more domains of cognitive function with plasma folate.
Although the number of participants was small, our study results clearly indicate that folate nutrition is related with cognitive function test scores for intelligence, memory attention, visuo-spatial abilities, and fine motor coordination in our elderly subjects. It may be difficult to apply what we found in our study directly to general pubic, however, there is growing evidence supporting the view that good nutritional status of folate and other B vitamins is an important determinant for cognitive function. It may be possible that some of the decline in cognitive function associated with aging is preventable or reversible with improved folate nutrition as suggested by Rosenberg et al. (1992). It is interesting to note, however, that study results on the effects of folic acid supplementation on cognitive function, dementia and Alzheimer's disease have not been consistent, some showing it to be beneficial (Bryan et al., 2002; Luchsinger et al., 2007; Nilsson et al., 2001), others showing a negligible effect (Baker et al., 1999; Wahlin et al., 2008) and still others showing it to be even detrimental (Sommer et al., 2003). Further studies should explore the relationship of the state of blood B vitamins to neuropsychological function and also the possibility of improving cognition with the dietary manipulation of natural sources of food folate.
Figures and Tables
Table 2
Nutritional state of thiamin, riboflavin, vitamin B6 and folate in subjects1)

1)Values are the mean ± S.E.
2)See references (Herbert, 1987; Leklem, 1990; Sauberlich et al., 1972; Tanphaichitr et al., 1970) for normal ranges of thiamin, riboflavin, vitamin B6 and folate status, respectively.
References
1. Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999. 113:13–20.

2. Baker H, De Angelis B, Baker ER, Frank O, Jaslowdagger SP. Lack of effect of 1 year of a high-dose vitamin and mineral supplement on cognitive function of elderly women. Gerontology. 1999. 45:195–199.

3. Baker SK, Tarnopolsky MA. Targeting cellular energy production in neurological disorders. Expert Opin Investig Drugs. 2003. 12:1655–1679.

4. Balaghi M, Horne DW, Wagner C. Hepatic one-carbon metabolism in early folate deficiency in rats. Biochem J. 1993. 1:145–149.

5. Bayoumi RA, Rosalki SB. Evalution of methods of coenzyme activation of erythrocyte enzymes for detection of deficiency of vitamins B1, B2, and B6. Clin Chem. 1976. 22:327.

6. Bell IR, Edman JS, Marby DW, Satlin A, Dreir T, Piptzin B, Cole JO. Vitamin B12 and folate status in acute geropsychiatric inpatients: affective and cognitive characteristics of vitamin nondeficient population. Biol Psychiatry. 1990. 27:125.

7. Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil micincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997. 94:3290–3295.

8. Bottiglieri T, Hyland K, Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994. 48:137–152.

9. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000. 69:228–232.

10. Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002. 132:1345–1356.

11. Buehring KU, Tamura T, Stockstad ELR. Folate coenzymes of Lactobacillus casei and Streptococcus faecalis. J Biol Chem. 1974. 249:1081.
12. Chen KJ, Pan WH, wang FL, Wei IL, Shaw NS, Lin BF. Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pac J Clin Nutr. 2005. 14:250–255.
13. Dakshinamurti K, Paulose CS, Siow YL. Reynolds RD, Leklem JE, editors. Neurobiology of pyridoxine. Vitamin B-6: Its Role in Health and Disease. 1985. New York. USA: Alan Riss, Inc.;99–121.

14. Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomized, double blind, controlled trial. Lancet. 2007. 369:208–216.

15. Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr. 2002. 75:908–913.

16. Fakhrzadeh H, Ghotbi S, Pourebrahim R, Nouri M, Heshmat R, bandarian F, Shafaee A, Larijani B. Total plasma homocysteine, folate and vitamin B-12 status in healthy Iranian adults: The Tehran homocysteine survey (2003-2004): a cross-sectional population based study. BMC Public Health. 2006. 6:29–37.
17. Feng L, Ng TP, Chuah L, Niti M, Kua EH. Homocysteine, folate, and vitamin B-12 and cognitive performance in older Chinese adults: findings from the Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2006. 84:1506–1512.

18. Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA. 1983. 249:2917.

19. Gospe SM Jr, Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, Ellis WG, Clifford AJ. Behavioral and neurochemical changes in folate-deficient mice. Physiol Behav. 1995. 58:935–941.

20. Haller J, Wegggemans RM, Ferry M, Guigoz Y. Mental health: Minimental state examination and geriatric depression score of elderly Europeans in the SENECA study of 1993. Eur J Clin Nutr. 1996. 50:S112–S116.
21. Hassing L, Wahlin A, Winblad B, Bäckman L. Further evidence on the effects of vitamin B12 and folate levels on episodic memory functioning: a population-based study of healthy very old adults. Biol Psychiatry. 1999. 45:1472–1480.

22. Heaton RK. A Manual for the Wisconsin Card Sorting Test. 1981. Odessa, FL. USA: Psychological Assessment Resources, Inc.
23. Herbert V. The 1986 Herman Award Lecture. Nutriton science as a continually unfolding : the folate and vitamin B-12 paradigm. Am J Clin Nutr. 1987. 48:387–402.
24. Hirata F, Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980. 209:1082–1090.

25. Joosten E, van den Berg A, Riezler R, Naurath HJ, Lindenbaum J, Stabler SP, Allen RH. Metabolic evidence that deficiencies of vitamin B-12 (cobalamine), folate, and vitamin B-6 occur commonly in elderly people. Am J Clin Nutr. 1993. 58:468–476.

26. LaRue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997. 65:20–29.

28. Lim HS, Heo YR. Plasma total homocysteine, folate and vitamin B-12 status in Korean adults. J Nutr Sci Vitaminol. 2002. 48:290–297.

29. Lindeman RD, Romero LJ, Koehler KM, Liang HC, LaRue A, Baumgartner RM, Garry PJ. Serum vitamin B-12, C and folate concentrations in the New Mexico elder health survey; correlations with cognitive and affective functions. J Am Coll Nutr. 2000. 19:68–76.

30. Luchsinger JA, Tang M-X, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007. 64:86–92.

31. Maj M, D'Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, Starace F, Galderisi S, Chervinsky A. Evaluation of three new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1-seropositive persons: a WHO study. Arch Clin Neuropsychol. 1993. 8:123.

32. Maxwell CJ, Hogan DB, Ebly EM. Serum folate levels and subsequent adverse cerebrovascular outcomes in elderly persons. Dement Geriatr Cogn Disord. 2002. 13:225–234.

33. Nilsson K, Gustafson L, Hultberg B. Improvement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteine. Int J Geriatr Psychiatry. 2001. 16:609–614.

34. Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005. 82:1346–1352.

35. Riggs KM, Spiro A III, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996. 63:306.

36. Rosenberg IH, Miller JW. Nutritional factors in physical and cognitive functions of elderly people. Am J Clin Nutr. 1992. 55:1237S–1243S.

37. Ruff RM, Parker SB. Gender- and age-specifics changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993. 76:1219.

38. Sauberlich HE, Judd JH, Nichoald GE, Broquist HP, Darby WJ. Application of the erythrocyte glutathione reductase assay in evaluating riboflavin nutritional status in a high school student population. Am J Clin Nutr. 1972. 25:756–762.

39. Scott JM, Molloy AM, Kennedy DG, Kennedy S, Weir DG. Effects of disruption of transmethylation in the central nerous system: an animal model. Acta Neurol Scand. 1994. 154S:27.
40. Sheu KF, Calingasan NY, Lindsay JG, Gibson GE. Immunochemical characterization of the deficiency of the alpha-ketoglutarate dehydrogenase complex in thiamine-deficient rat brain. J Neurochem. 1998. 70:1143–1150.

41. Smith AD. Homocysteine, B vitamins, and cognitive deficit in the elderly. Am J Clin Nutr. 2002. 75:785–786.

42. Snowdon DA, Tully CL, Smith CD, Riley KP, Markesbery WR. Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: findings from the Nun Study. Am J Clin Nutr. 2000. 71:993–998.

43. Sommer BR, Hoff AL, Costa M. Folic acid supplementation in dementia: a preliminary report. J Geriatr Psychiatry Neurol. 2003. 16:156–159.

44. Tanphaichitr B, Vimokesant SL, Dhanamitta S, Valyasevi A. Clinical and biochemical studies of adult beriberi. Am J Clin Nutr. 1970. 23:1017–1026.

45. Tettamanti M, Garrì MT, Nobili A, Riva E, Lucca U. Low folate and the risk of cognitive and functional deficits in the very old: the Monzino 80-plus study. J Am Coll Nutr. 2006. 25:502–508.

46. Varela-Moreiras G, Selhub J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992. 122:986–991.

47. Wagner C. Bailey LB, editor. Biochemical role of folate in cellular metabolism. Folate in Health and Disease. 1995. New York. USA: Marcel Dekker, Inc.;23–43.

48. Wahlin A, Fahlander K, Wahlin T-B, Bunce D, Backman L. Vitamin B status and cognitive perfomance in preclinical and clinical Alzheimer's disease: Data form The Kungsholmen Project. Dementia and Geriatr Cogn Disord. 2008. 25:23–31.

49. Wahlin A, Hill RD, Winblad B, Backman L. Effects on serum vitamin B12 and folate status on episodic memory performance in very old age: a population-based study. Psychol Aging. 1996. 11:487–496.

50. Wechsler D. WAIS-R Manual. 1981. San Antonio, TX. USA: The Psychological Corporation.
51. Wechsler D. Wechsler Memory Scale-Revised Manual. 1987. San Antonio, TX. USA: The Psychological Corporation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download