Abstract
The matrix metalloproteinases (MMP) play an important role in tumor invasion, angiogenesis and inflammatory tissue destruction. Increased expression of MMP was observed in benign tissue hyperplasia and in atherosclerotic lesions. Invasive cancer cells utilize MMP to degrade the extracellular matrix and vascular basement membrane during metastasis, where MMP-2 has been implicated in the development and dissemination of malignancies. The present study attempted to examine the antiangiogenic activity of the medicinal herbs of Aspergillus usamii var. shirousamii-transformed Angelicae Gigantis Radix and Zizyphus jujube (tAgR and tZj) with respect to MMP-2 production and endothelial motility in phorbol 12-myristate 13-acetate (PMA)- or VEGF-exposed human umbilical vein endothelial cells (HUVEC). Nontoxic tAgR and tZj substantially suppressed PMA-induced MMP-2 secretion. In addition, 25 µg/mL tAgR and tZj prevented vascular endothelial growth factor-stimulated endothelial cell transmigration and tube formation. The results reveal that tAgR and tZj dampened endothelial MMP-2 production leading to endothelial transmigration and tube formation. tAgR and tZj-mediated inhibition of endothelial MMP may boost a therapeutic efficacy during vascular angiogenesis.
Angiogenesis is important for vascular remodeling in the embryo as well as in female reproductive cycles and wound healing in the adult (Eming et al., 2007). However, aberrant angiogenesis occurs under certain pathological conditions such as rheumatoid arthritis, diabetic retinopathy, psoriasis, hemangiomas, and cancer (Battegay, 1995).The signals that initiate and sustain angiogenesis are multiple and complex, in which proangiogenic growth factors and cytokines including vascular endothelial growth factors (VEGF), platelet-derived growth factors, angiopoietins and interleukin-8 are involved (Li et al., 2003; Liekens et al., 2001). These factors and cytokines are secreted by inflammatory cells, pericytes, keratinocytes or tumor cells. Some of these factors act directly by binding to respective receptors on endothelial cells to induce proliferation and/or migration, while others act on local stromal or inflammatory cells to stimulate angiogenesis (Weinstat-Saslow & Steeg, 1994). Accordingly, an understanding of normal and abnormal angiogenesis is required in order to develop therapeutic strategies to combat these pathologies (Stetler-Stevenson, 1999). Since angiogenesis involves migration or invasion of endothelial cells into surrounding stroma/tissues, proteases such as the matrix metalloproteinases (MMP) are pivotal (Li et al., 2003). Various MMP degrade the extracellular matrix (ECM) to facilitate invading endothelial cells (Stamenkovic, 2003; Stetler-Stevenson, 1999). In addition, MMP are necessary for releasing ECM-sequestered proangiogenic factors, processing growth factors and receptors, and for generating endogenous antiangiogenic compounds (Liekens et al., 2001).
Angelicae Gigantis Radix (AgR), Korean Angelica gigas Nakai (Dang Gui) root, is one of the most widely used herbal traditional medications for anemia or blood circulatory disorders (Yun et al., 2007). A recent study showed that AgR had an inhibitory effect on the osteoclast formation partially through the NF-κB pathway (Kil et al., 2008). AgR ethanol extract inhibited melanogenesis in B16 melanoma cells with the downregulation of tyrosinase expression via a cAMP-dependent pathway (Lv et al., 2007). Zizyphus jujuba (Zj) has been reported to have effects of neuronal stabilization (Park et al., 2004). Jujuboside A is a main component of Jujubogenin extracted from Zj seeds, which is used in traditional medicine for the treatment of insomnia and anxiety (Zhang et al., 2003). It was shown that Jujuboside A exerts inhibitory effects on glutamate-mediated excitatory signal pathway in hippocampus through its anti-calmodulin action (Zhang et al., 2003). Additionally, saponins extracted from Zizyphus jujube seeds had potent sedative and hypnotic functions (Jiang et al., 2007).
On the basis of the literature evidence, the medicinal herbs of AgR and Zj are beneficially effective in treating various diseases of osteoporosis, hyperpigmentation or insomnia (Kil et al., 2008; Lv et al., 2007; Zhang et al., 2003). Nevertheless, microbial transformations in the gut can affect their bioactivity. The present study assessed the antiangiogenic activity of the medicinal herbs of Aspergillus usamii var. shirousamii (A. usamii)-transformed AgR and Zj (tAgR and tZj) with respect to MMP production and endothelial motility in phorbol 12-myristate 13-acetate (PMA)- or VEGF-exposed human umbilical vein endothelial cells (HUVEC).
Crude microbial enzyme extracts of the microbial A. usamii were prepared according to a previously described method with a minor modification (Chi et al., 2005). A. usamii KCTC 6956 was purchased from the Korean Collection for Type Cultures (Daejeon, Korea). The collected spores of subcultured A. usamii were suspended in a suspension buffer (0.9% NaCl solution with 0.005% Tween 80) at 1×107 spores/mL density and inoculated in Malt Extract broth (Difco Laboratories, Becton Dickinson, MD). The culture broth was incubated at 24℃ for 4 d under aerobic conditions with shaking. The concentrate of the collected medium was used as a crude enzyme extract for the transformation of Angelica gigas Nakai and Zizyphus jujuba. The mycelia of A. usamii was removed by filtration using glass microfiber filter (GF/A, Whatman, Kent, UK), and the filtrate was treated with 80% (w/v) (NH4)2SO4. The resulted precipitate was collected by centrifugation at 12,000xg for 30 min at 4℃ and dissolved in 50 mM phosphate buffer (pH 6.0), followed by dialysis (10 KD-MW cut off) against the same buffer. The dialyzed protein extracts were used as microbial crude enzyme sources for the transformation of AgR and Zj extracts.
Microbial transformation using crude microbial enzyme extracts was conducted as previously described (Chi et al., 2005). AgR and Zj were purchased from oriental drug stores, Kyeong-Dong market (Seoul, Korea). Fifty grams of AgR or Zj were immersed in 10 volumes of distilled water, kept overnight at 4℃ and extracted twice at 60℃ for 3 h at 120 rpm in the shaking water bath. The water extracts were sonicated at 40Hz for 1 h, filtered and lyophilized.
The lyophilized extracts of AgR or Zj were dissolved in 50 mM phosphate buffer (pH 6.0) at a concentration of 20 mg/mL, mixed with same volume of prepared crude enzyme extracts of A. usmii, and incubated at 37℃ for 24 h. Before mixing, the plant extracts and crude microbial enzyme extracts were filtered to avoid any contamination. The reaction was terminated by boiling the reaction mixtures in water for 30 min and frozen at -80℃ before use.
HUVEC were isolated from umbilical cords using collagenase as described elsewhere (Choi et al., 2005). Endothelial cells were cultured in 25 mM HEPES-buffered M199 containing 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin supplemented with 0.75 mg/mL human epidermal growth factor and 0.075 mg/mL hydrocortisone and cultures were maintained at 37℃ humidified atmospheres of 5% CO2 in air.
The cytotoxicity of tAgR and tZj in the presence of PMA was examined using 3-(4,5-dimetylthiazol-yl)-diphenyl tetrazolium bromide (MTT) (Choi et al., 2003). At the end of the incubation with 2H and 8H in the presence of PMA, the MTT assay was performed to quantitate cellular viability. HUVEC were incubated in a fresh medium containing 1 mg/mL MTT for 3 h at 37℃. After removal of unconverted MTT, the purple formazan product was dissolved in 0.5 mL isopropanol through gentle shaking. Absorbance of formazan dye was measured at λ=570 nm using a microplate reader.
HUVEC were plated at 90-95% confluence in all experiments. Cells were incubated overnight with 1-25 µg/mL 2H and 8H prior to exposing to 50 ng/mL PMA added to serum-free HEPES-buffered M199. Gelatin zymography for the measurement of MMP-2 activity was performed as previously described (Kargozaran et al., 2007). Briefly, culture supernatants were subjected to electrophoresis on 10% SDS-PAGE in a Tris-HCl buffer [0.3 M Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, and 0.03% bromophenol blue] co-polymerized with 0.1% gelatin as the substrate. After electrophoresis was complete, the gel was incubated for 1 h at 37℃ in a 2.5% Triton X-100, washed in 50 mM Tris-HCl buffer (pH 7.5) for 30 min, and incubated for 20 h in 50 mM Tris-HCl buffer (pH 7.5) containing 200 mM NaCl, 10 mM CaCl2 and 0.05% Brij-35. The gel was stained with 0.1% Coomassie Brilliant Blue G-250, 2% acetic acid and 45% methanol, and then destained in a solution with 30% methanol and 10% acetic acid.
The cell transmigration assay was performed in an 8-µm pore transwell chamber. HUVEC were re-suspended in a serum free medium containing 5 ng/mL VEGF and seeded at 60,000 cells per well on gelatin-coated filters. Cells transmigrated onto the lower surface of the filter were stained with toluidine blue, and cells migrated for 24 h were counted and photographed in the 2-3 microscopic fields per well using a microscope with CCD camera (Motic®, Wetzlar, Germany).
The Matrigel basement membrane Matrix (BD Biosciences, Heidelberg, Germany) was used as substrate for the in vitro study of angiogenesis. The Matrigel was thawed overnight at 4℃, homogenously mixed in a culture medium, and allowed to solidify in a sterile environment for 24 h. HUVEC were harvested by trypsinization, and 100,000 cells per well were seeded onto 24-well plates on which 100 µL Matrigel (1:1 dilution) was distributed. Cells were incubated for 1 h at 37℃ to gelatinize. The branching points were continuously monitored and the tube formation of cells was photographed in five random fields of view per well using a Motic microscope.
Values were represented as means ± SEM of separate experiments. Statistical analyses were conducted using Statistical Analysis Systems statistical software package (SAS Institute Inc., Cary, NC). Significance was determined by one-way ANOVA followed by Duncan's multiple range test for multiple comparisons. The P-values less than 0.05 were considered as statistically significant.
To examine cytotoxicity of tAgR and tZj on human endothelial cells, various concentrations of tAgR and tZj in a range of 1-25 µg/mL were added to PMA-treated cells for 24 h, and cell viability was measured by MTT analysis. When tAgR in concentrations between 1 and 25 µg/mL was added to PMA-exposed HUVEC, the viability was not affected. In addition, tZj did not show any toxic effects (Fig. 1B). Accordingly, tAgR and tZj were used at ≤25 µg/mL nontoxic concentrations for the culture experiments.
In order to determine inhibitory effects of tAgR and tZj on production of MMP-2, gelatin zymography was assayed. PMA exposure for 24 h substantially elicited MMP-2 production in serum-free conditions (Fig. 2). When PMA-exposed cells for 24 h were treated with 25 µg/mL tAgR, the proteolytic activity of MMP-2 was significantly attenuated (Fig. 2A). Additionally, it was found that tZj at 25 µg/mL dampened the MMP-2 secretion enhanced by PMA (Fig. 2B).
An inhibitory role of tAgR and tZj in the endothelial cell motility was assessed using a transmigration assay. Both 25 µg/mL tAgR and tZj showed no endothelial cytotoxicity in the presence of VEGF, as evidenced by MTT assay (data not shown). This study investigated whether 25 µg/mL tAgR and tZj blocked the VEGF-activated transmigration of HUVEC cultured on the transwell chamber with a gelatin-coated filter. The addition of VEGF to HUVEC significantly promoted endothelial transmigration (Fig. 3). In contrast, 25 µg/mL tAgR and tZj significantly abolished transmigration in VEGF-stimulated endothelial cells. The production of MMP to degrade ECM and basement membrane is pivotal in the endothelial cells. In this study, the MMP-2 data obtained from the gelatin zymography (Fig. 2) supported the transmigration data (Fig. 3).
Next, it was tested using a tubular morphogenesis assay whether tAgR and tZj modulated VEGF-induced HUVEC tube formation. As expected, VEGF led to the formation of tube-like structures after its 12 h-treatment (Fig. 4). When 25 µg/mL tAgR or tZj was added to cells treated with VEGF, the tube formation effectively was diminished. Accordingly, tAgR and tZj appeared to inhibit endothelial cell organization into the vascular network through new vessel formation under inflammatory and tumorigenic conditions.
Three major findings were observed in this study. 1) When tAgR and tZj at the non-toxic dose of 25 µg/mL was added to endothelial cells, the gelatinolytic activity of MMP-2 elevated by PMA were substantially mitigated, indicating that both tAgR and tZj can inhibit MMP production. 2) VEGF enhanced the endothelial transmigration, which was reversed by 25 µg/mL tAgR and tZj. 3) VEGF-stimulated endothelial tube formation was dampened by an addition of 25 µg/mL tAgR or tZj. It was deemed that the microbial transformation of AgR and tZj blocked the production of MMP-2 to degrade ECM and basement membrane, which might in turn modulate endothelial cell motility and capillary-like endothelial tube formation.
An herbal decoction prepared from AgR has been used as a traditional herbal traditional medication for the treatment of menopausal symptoms (Haines et al., 2008). It was shown that AgR had an inhibitory effect on the osteoclast formation partially through the NF-κB pathway (Kil et al., 2008). AgR inhibited the melanogenesis of melanoma cells with attenuating tyrosinase expression via cAMP-dependent pathway (Lv et al., 2007). In addition, an herbal decoction prepared from Radix Astragali and Angelicae induced the expression of erythropoietin in cultured Hep3B cells (Gao et al., 2008). A recent report showed that extracts from the herb plants Angelicae and Chuanxiong could affect VEGF expression in rat myocardial infarction, promote endothelial cell proliferation and stimulate quantity of vessels in chick embryo chorioallantoic membrane models, indicating that these herbs had angiogenic effects as target mechanisms for the treatment of myocardial infarction and peripheral ischemia (Meng et al., 2008). The pyranocoumarin compound decursin from an oriental formula containing AgR has been reported as a novel anti-androgen receptor signaling agent (Lu et al., 2007).
Zizyphus jujuba (Zj) is another traditional medicinal herb and has neuronal stabilization activity (Park et al., 2004). Antistress and insomnia formula based on Ziziphus are well-documented sedative properties (Wang et al., 2008; Zhang et al., 2003). Thus, Zizyphus is of particular benefit for menopausal insomnia and nerve control of men and women of all ages. In particular, flavonoids, saponins, and polysaccharides extracted from Semen Zj had sedative and hypnotic functions (Jiang et al., 2007). In addition, sanjoinine A, one of major alkaloid compounds of Zizyphi Semen, has hypnotic effects and enhances pentobarbital-induced sleeping behaviors through the γ-aminobutyric acid systems (Ma et al., 2007).
Due to the success of therapeutic functions to reverse the development of menopausal symptoms and insomnia, the identification of these medicinal herbs and the purification of their constituents are being vigorously pursued. However, it is deemed that the metabolic transformation in the gut may affect their bioactivity. Commonly consumed herbal decoction has the potential to undergo predominant microbial transformations. The present study mimicked the microbiota-induced metabolic transformation of AgR and Zj in the gut. The potential antiangiogenic properties were determined by measuring the ability of AgR and Zj to modulate endothelial motility and capillary-like tube formation stimulated following a PMA-or VEGF-induced insult. The microbial transformation of AgR and Zj may have important therapeutic implications in the prevention of certain pathological diseases such as rheumatoid arthritis, diabetic retinopathy, psoriasis, hemangiomas, and cancer. The resultant metabolites of tAgR and tZj may have differing effects on angiogenesis from untransformed parent AgR and Zj ranging from a slight increase to a significant reduction in magnitude. Unfortunately, we did not examine the antiangiogenic activity of parent AgR and Zj.
Crude microbial enzyme extracts prepared by A. usamii would allow a specific bioconversion process to obtain specific metabolites via appropriate combination of AgR and Zj. An appropriate combination of protopanaxadiol ginsenosides of Rb2 and Rc and specific enzymes extracted from various edible food microorganisms were converted into specific ginsenosides (Chi et al., 2005). This study did not conduct a p-nitophenol assay to determine the β-glucosidase activity of crude enzyme extracts prepared from the cultured A. usamii. However, it is speculated that flavonoid glycosides, saponins, and polysaccharides possibly present in AgR and Zj are involved in the microbial transformation. Spinosin, a C-glycoside flavonoid of semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep in mice via a serotonergic mechanism (Wang et al., 2008). Microbial transformation with Sepedonium chrysospermem of decursin yielded two metabolites, decursinol and cis-decursidinol (Herath et al., 2007). It was shown that decursinol may act as a chemopreventive agent to suppress the uncontrolled growth and invasive potential, angiogenic and metastatic targets, of prostate cancer (Singh & Agarwal, 2006).
In summary, the present study has shown that microbial biotransformation of AgR and Zj used for medicinal herbal decoction had therapeutic implications in pathological diseases pertaining to aberrant angiogenesis. Nontoxic tAgR and tZj suppresses gelatinolytic MMP-2 activity upregulated by PMA, which was responsible for blocking endothelial transmigration and tube formation most likely through degrading the basement membrane. Compelling evidence is drawn from this study that tAgR and tZj has the potential capability to prevent angiogenesis, inflammation and atherosclerosis involving ECM degradation.
Figures and Tables
Fig. 1
Cytotoxicity of tAgR (A) and tZj (B) in PMA-exposed HUVEC. After HUVEC were cultured for 24 h in the presence of 50 ng/mL PMA and 1-25 µg/mL tAgR and tZj, MTT assay was performed. The bar graph data represent means ± SEM from 5 independent experiments with multiple estimations. Values are expressed as percent cell survival relative to untreated control cells (cell viability=100%). *P<0.05, compared to untreated control.
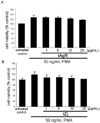
Fig. 2
Gelatinolytic activity of MMP-2 of PMA-exposed HUVEC treated with 1-25 µg/mL tAgR (A) and tZj (B). After HUVEC culture protocols with 50 ng/mL PMA and 1-25 µg/mL tAgR and tZj for 24 h, equal volumes of culture media were subjected to 10% SDS-PAGE for the measurement of the enzyme activity of MMP-2. Gelatinolytic activity was detected as unstained bands against the background of Coomassie blue-stained gelatin. The bar graphs (means ± SEM, n=4) represent quantitative densitometric results of upper bands. *P<0.05, compared to untreated control. †P<0.05, compared to untreated control and 50 ng/mL PMA.
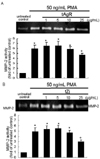
Fig. 3
Inhibition of cell transmigration by tAgR and tZj in VEGF-treated endothelial cells. HUVEC were cultured on gelatin-coated filters of 8 µg/mL pore transwells. Cells transmigrated for 24 h onto the lower surface of the filter were stained with toluidine blue and counted. The bar graphs (means ± SEM, n=4) represent the numbers of cells transmigrated. *P<0.05, compared to untreated control.
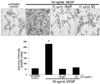
Fig. 4
Representative images showing tube formation in VEGF-treated endothelial cells plated onto matrigel. After 12 h incubation with 25 µg/mL tAgR and tZj, cells were fixed and images at ×100 magnification were captured. The number of tubes from the images was counted. Multiple five random fields of view were analyzed for the quantitative results. Tube formation results are plotted as means ± SEM (n=4). *P<0.05, compared to VEGF-untreated control.

Notes
This study was supported by the 21C Frontier Microbial Genomics and Applications Center Program, Ministry of Science and Technology (MG08-0303-4-0), Korea, the research grant from the Small and Medium Business Administration (S0807222-G0942940-10100013, 2008), Korea and supported by the Korea Research Foundation Brain Korea 21.
References
1. Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995. 73:333–346.

2. Chi H, Kim DH, Ji GE. Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull. 2005. 28:2102–2105.

3. Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005. 135:707–713.

4. Choi YJ, Kang JS, Park JH, Lee YJ, Choi JS, Kang YH. Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. J Nutr. 2003. 133:985–991.

5. Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007. 42:115–170.

6. Gao QT, Cheung JK, Choi RC, Cheung AW, Li J, Jiang ZY, Duan R, Zhao KJ, Ding AW, Dong TT, Tsim KW. A Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis induces the expression of erythropoietin in cultured Hep3B cells. Planta Med. 2008. 74:392–395.

7. Haines CJ, Lam PM, Chung TK, Cheng KF, Leung PC. A randomized, double-blind, placebo-controlled study of the effect of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) on menopausal symptoms in Hong Kong Chinese women. Climacteric. 2008. 11:244–251.

8. Herath W, Reddy N, Khan IA. Microbial metabolism. The pyranocoumarin, decursin. Chem Pharm Bull (Tokyo). 2007. 55:1512–1513.
9. Jiang JG, Huang XJ, Chen J, Lin QS. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat Prod Res. 2007. 21:310–320.

10. Kargozaran H, Yuan SY, Breslin JW, Watson KD, Gaudreault N, Breen A, Wu MH. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis. 2007. 24:495–502.

11. Kil JS, Kim MG, Choi HM, Lim JP, Boo Y, Kim EH, Kim JB, Kim HK, Leem KH. Inhibitory effects of Angelicae Gigantis Radix on osteoclast formation. Phytother Res. 2008. 22:472–476.

12. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003. 60:107–114.

13. Liekens S, De Clercq E, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol. 2001. 61:253–270.

14. Lu J, Kim SH, Jiang C, Lee H, Guo J. Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents. Acta Pharmacol Sin. 2007. 28:1365–1372.

15. Lv N, Koo JH, Yoon HY, Yu J, Kim KA, Choi IW, Kwon KB, Kwon KS, Kim HU, Park JW, Park BH. Effect of Angelica gigas extract on melanogenesis in B16 melanoma cells. Int J Mol Med. 2007. 20:763–767.

16. Ma Y, Han H, Eun JS, Kim HC, Hong JT, Oh KW. Sanjoinine A isolated from Zizyphi Spinosi Semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biol Pharm Bull. 2007. 30:1748–1753.

17. Meng H, Guo J, Sun JY, Pei JM, Wang YM, Zhu MZ, Huang C. Angiogenic effects of the extracts from Chinese herbs: Angelica and Chuanxiong. Am J Chin Med. 2008. 36:541–554.

18. Park JH, Lee HJ, Koh SB, Ban JY, Seong YH. Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi spinosi semen in cultured rat cerebellar granule cells. J Ethnopharmacol. 2004. 95:39–45.

19. Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006. 13:751–778.

20. Stamenkovic I. Extracellular matrix remodeling: The role of matrix metalloproteinases. J Pathol. 2003. 200:448–464.
21. Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J Clin Invest. 1999. 103:1237–1241.

22. Wang LE, Bai YJ, Shi XR, Cui XY, Cui SY, Zhang F, Zhang QY, Zhao YY, Zhang YH. Spinosin, a C-glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol Biochem Behav. 2008. 90:399–403.

23. Weinstat-Saslow D, Steeg PS. Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J. 1994. 8:401–407.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download