Abstract
This study was focused on whether or not isoflavones affect the increase in bone mineral density of growing females. Female Sprague-Dawley rats (60 ± 5 g) were randomly assigned to two groups and provided control diets (control group) or isoflavone-supplemented diet (IF group, 57.8 mg isoflavones/100 g diet) for 9 weeks in growing female rats. Measurements of Bone Mineral Density (BMD) and Bone Mineral Content (BMC) on the experimental animals were executed in the 3rd, 6th, 9th weeks. In result, there was no significant difference in spine BMD between the isoflavones supplemented group and the control group. But, the IF group tended to have higher BMD than the control group in between 3 and 9 experimental weeks, and the striking difference could be shown in the 6th week of feeding. In case of femur BMD, the effects of added isoflavones appeared in the 6th week of feeding, and it became intensified in the 9th week of feeding to the extent that the BMD in the IF group was significantly higher than that of the control group (p<0.05). In conclusion, isoflavone supplementation increased spine BMD per weight in the 6th week of feeding, and affected the increase of femur BMD in the 9th week. The result of the experiment implies that it affects positively the formation of spine and femur BMD of growing female rats. The study also suggests that the effects of isoflavone on the pattern of BMD formation might differ from the parts of bones.
Go to : 
Epidemiological studies suggest that the low incidence of osteoporosis and heart diseases caused by estrogen deficiency in Asian women is attributable to their high intake of soy foods, compared with American and Finnish women (Adlercreutz et al., 1992; Anderson et al., 1995; Brandi, 1997). It is reported that dietary soybean proteins prevent bone loss in ovariectomized (OVX) rats (Arjmandi et al., 1996). Possible candidates for the beneficial substances present in soybeans are isoflavones, such as genistein and daidzein (Ishimi et al., 1999). Isoflavones are estrogen-like substances structurally and functionally similar to 17 β-estradiol (Knight & Eden, 1996). On the basis of evidence primarily from animal and in vitro studies, isoflavones are thought to exert both estrogenic and antiestrogenic effects, depending on the tissue in which they act (Makela et al., 1994). Isoflavones may exert a weak antagonistic effect on the estrogen receptor (Makela et al., 1994), thereby having an antiestrogenic effect on uterine and breast tissue (Santell et al., 1994), where excess estrogen may stimulate synthesis. Alternatively, isoflavones may combine with the estrogen receptor, albeit with lower affinity than 17 β-estradiol (Miksicek, 1994), and stimulate estrogen activity, thus having an estrogenic effect on bone (Makela et al., 1994) and blood vessels (Schonherr et al., 1997).
Recent studies (Eriksen et al., 1998; Oursler et al., 1991) have shown that phytoestrogen has a higher binding affinity to estrogen receptor-β than to estrogen receptor-α. It is suggested that the possibility of isoflavones having a tissue-selective effect is high, since isoflavones may function more selectively on such organs as the thyroid gland, bones and blood vessels, where estrogen receptor-β is highly dispersed (Kuiper et al., 1998).
Some in vitro studies have suggested that isoflavones may have anti-estrogenic effects and estrogenic effects at the same time (Jayagopal et al., 2002; Lees & Ginn, 1998). The studies report that isoflavones may not be good for growing and young women, for they would reduce the activation of endogenous estrogen (Jayagopal et al., 2002).
On the contrary, according to some recent studies, isoflavone intake in infancy through soy-based infant formulas may have positive effects in the long term, as it may prevent hormone-dependent diseases such as cancer, osteoporosis, cardiovascular diseases, etc. that can be developed in the latter part of adulthood (Setchel et al., 1998). Disputes over the theory still remain unsettled, though. There is no concrete result on whether or not the isoflavones found in soy protein cause a decline in bone density, or osteoporosis. There is little evidence of the mechanism, either. Since most previous studies on isoflavones focused on the features and effects of isoflavones that are similar to those of estrogen, the target of the experiments were pre- or post-menopausal women and ovariectomized animals.
It has been rarely studied if isoflavones have positive or negative effects on growing and young women. Whatever studies have been conducted has shown no significant results yet. The most important risk factor of the occurrence of osteoporosis is low peak bone mass and rapid rates of bone loss. As such, mitigation of the risk of fracture is dependent on an increase in the peak bone mass and thereafter, on minimization of bone loss.
This study was focused on whether or not isoflavones-supplementation affects the increase in bone mineral density of growing females. To examine the effects of isoflavones, we provided isoflavone which is extracted from soy for 9 weeks to growing female rats.
Go to : 
Female Sprague-Dawley rats (60 ± 5 g) were bought from KLEC (Korea Life Engineering Co., Seoul, Korea). Rats were fed stock diets (rat chow made by Samyangsa) for a week of adaptation period. Then, they were randomly divided into two experimental dietary groups, which included 12 rats each, and provided experimental diets for 9 weeks.
All experimental rats were individually housed in stainless steel wired cage in an air-conditioned room with controlled temperature (25 ± 2℃) and humidity (63 ± 5%) and automatic lighting (alternation 12-h period of light and dark). The experimental diet and deionized water were provided ad libitum.
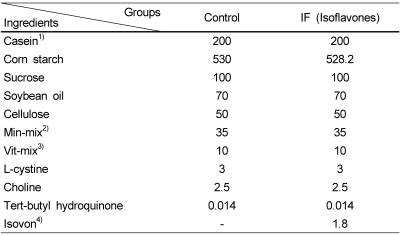
The experimental groups were divided into control group and isoflavone supplemented group (IF). The diets were formulated based on AIN-93G (Reeves et al., 1993). The two groups were based on same composition, but the diet of IF group was provided with isoflavones, extracted from soybean. The amount of isoflavone supplemented into experimental diet was 57.8 mg /100 g diet. The composition of experimental diets is shown in Table 1.
During the experiment period, the amount of dietary intake was measured once every other day and the weight of experimental animals, once a week at a specific time.
Measurements of bone mineral density (BMD) and bone mineral content (BMC) on the experimental animals were executed in the 3rd, 6th, 9th weeks. After the rats received intramuscular injection (75 mg/kg) with ketamine hydrochloride (Yuhan Kimberly, 50 mg/ml), their BMD and BMC in their spine and femur were measured with PIXImus, which is specialized for small animal, and with Dual energy x-ray absorptiometry (DEXA) made by GE, LUNAR (Madison, WI, USA). The unit of BMC is gram (g), and that of BMD is gram per square centimeter (g/cm2).
Statistical analysis was conducted using the SAS program (Statisical Analysis System 9.13 version, SAS Institute Inc. Cary, NC). The mean and standard deviation were calculated for all the variables, statistical significance between the groups was tested by t-test. Differences were considerd significant at p<0.05.
Go to : 
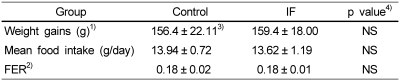
Weight gains, mean food intake, and food intake efficiency ratio (FER) observed for 9 weeks are shown in Table 2. Supplements of isoflavones had no significant effects on weight gains, mean food intake, and FER.
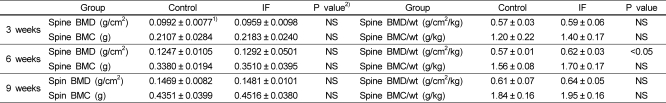
After 3, 6 and 9 weeks of feeding experimental diets, the results of spine BMD, BMC, BMD per weight, and BMC per weight are shown in Table 3. There was no significant difference between control group and IF group in spine BMD and BMD per weight after 3 weeks of feeding. The results of BMC and BMC per weight were not significantly different between control and IF groups in the 3rd week.
After 6 weeks of feeding, as spine BMD in control group was 0.1247 g/cm2, and IF group was 0.1292 g/cm2, there was no significant difference between the two groups. The amount of spine BMD increase was 0.0255 g/cm2 in the control group and 0.0333 g/cm2 in IF group between the 3rd and 6th weeks of feeding, showing that IF group showed 30.6% more increase than control group.
Spine BMD per weight in IF group (0.62 g/cm2/kg) was significantly higher than that in control group (0.57 g/cm2/kg). The difference between groups was 8.8%, and it has significant meaning. This indicates much higher increase in IF group than in control group. Spine BMC between the two groups had no significant difference in the 6th week. The increase in spine BMC between 3rd week and 6th week was 0.1273 g in the control group and 0.1327 g in the IF group. Spine BMC per weight of IF group was 8.9% higher than control group in the 6th week, but it was not significantly different.
After 9 weeks of feeding, there was no significant difference between control group (0.1469 g/cm2) and IF group (0.1481 g/cm2) in spine BMD. Spine BMC in the 9th week was 0.4351 g in control group and 0.4516 g in IF group respectively and the data showed no significant difference. In the 9th week, there was no significant difference between groups in BMD and BMC per weight, but IF group tended to have higher figures than control group.
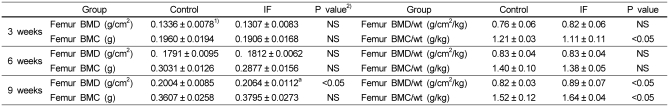
After 3, 6 and 9 weeks of feeding experimental diets, the results of femur BMD, BMC, BMD per weight and BMC per weight are shown in Table 4. After 3 weeks of feeding, femur BMD was 0.1336 g/cm2 in control group and 0.1307 g/cm2 in IF group respectively. It showed no significant difference. Femur BMC with adding isoflavone in the period indicated no difference as well. Femur BMD per weight after 3 weeks of feeding between groups showed no significant difference, but BMC per weight in IF group was significantly lower than that in control group.
After 6 weeks of feeding, femur BMD was not significantly different between control group (0.1791 g/cm2) and IF group (0.1812 g/cm2). However, the amount of growth on femur BMD growth was 0.0456 g/cm2 in the control group and 0.0505 g/cm2 in IF group between the 3rd and 6th weeks of feeding, showing that IF group had 10.7% more growth than control group. Femur BMC in the two groups indicated no significant difference in the 6th weeks of feeding. After 6 weeks of feeding, femur BMD per weight and femur BMC per weight in this period showed no significant difference.
Different from the results of the 3rd and 6th weeks, the femur BMD of IF group was significantly higher than that of control group after 9 weeks. Femur BMD per weight of IF group was 0.89 g/cm2/kg, which was significantly higher than that of control group. Femur BMC measured after 9 weeks as well, in IF group had higher figures than control group, but there was no significant difference. Tendency of femur BMC in IF group was lower than that of control group in the 3rd and 6th weeks, but the former became higher than the latter in the 9th week. The amount of growth on femur BMC was 0.1647 g/cm2 in the control group and 0.1889 g in IF group between the 3rd and 9th weeks of feeding, showing that IF group had 14.7% more increase than control group. In the 9th week of feeding, Femur BMC per weight of IF group was significantly higher than that in control group as well.
Go to : 
In this study, isoflavones had no significant effects on weight gains, mean food intake, and FER. In previous studies (Choi & Cho, 2003), both soy isolate and soy concentrate groups showed significantly lower in FER than casein group. This indicates that there is no difference in FER according to isoflavone intake groups but varies according to protein sources.
In this study, the spine BMD of control group showed 0.0992 g/cm2 (3rd weeks), 0.1247 g/cm2 (6th weeks), and 0.1469 g/cm2 (9th weeks). The spine BMD in male rats of similar weeks of ages was reported 0.15 g/cm2 in the study of Chae (2002). The result is much similar to that of this study, 0.1469 g/cm2. This study observed how and how much soy protein which contains high isoflavones and isoflavones itself affected spine BMD and BMC in the growing female rats. One of our findings are that the increased rate of spine BMD in the week of 3 to 6 was much higher than in the week of 6 to 9 after feeding.
Especially, spine BMD increase of the IF group in the week 3 to 6 (0.0302 g/ cm2) was 37.4% more than that in the week 6 to 9 (0.0189 g/cm2). In general, SD species white rats ovulate regularly after 8 weeks of age and secrete estrogen stably after 13 weeks of age. In regarding this, since the secretion of estrogen was relatively low in between the 3rd week of experiment (8~9 weeks of age) and the 6th week of experiment (11~12 weeks of age), the effects of isoflavones can be effective in spine that has more trabecula bone than femur. According to the previous study (Choi & Cho, 2003) that reported the effects of soy protein on the BMD of growing female rats by adding same amounts of isoflavones as in this study, the spine BMD in the isoflavones-supplemented group in the 3rd week of experiment was significantly higher than that of casein group. The result of the former group was significantly higher in the 6th week as well, but not statistically significant in the 9th week.
The pattern of increasing spine BMD is similar to the previous study that supplemented isoflavones-abundant soy protein, but the extent of the effects on spine BMD increase is not identical. Isoflavones-abundant soy protein affected the increase of spine BMD than extracted isoflavones did, and the difference with the control group was bigger. Considering IF group affects spine BMD relatively weak, the isoflavones effect on spine BMD seems not only by isoflavones itself.
Compared this study with previous animal studies on the level of BMD, which measured by PIXImus on male rats of similar weeks of ages in this study, the result of femur BMD was 0.23 g/cm2 and BMC was 0.54 g (Chae, 2002). The level is somewhat higher than those of this study measured on female rats. Femur BMD and BMC measured by DEXA on male rats in 4 weeks of age were reported as 0.25-0.26 g/cm2 and 0.37-0.49 g, respectively. Griffin et al. (1993) reported that the femur BMD of the SD species white rats in 2~8 weeks of age was 0.30~0.32 g/cm2. With regard to those results, BMD of the experimental animals mostly relies on the week of ages of the samples and the kinds of measurements.
The study observed periodically, classified by diet, the effects of isoflavones-abundant soy protein and isoflavones on femur BMD and BMC in growing age. The femur BMD in the 3rd and 6th week had no significant difference between the two groups. In the 9th weeks of feeding, since the increase rate of femur BMD had become higher, IF group tended to have bigger femur BMD than other groups. However, the increase of BMD in IF group became much higher after 9 weeks of feeding, and the difference from control group becomes significant. between the 3rd and 9th weeks of feeding.
The growth patterns of spine BMD and femur BMD in the 9th weeks of feeding have some differences. While the difference between the two experimental groups in spine BMD was not significant, IF group in the 3rd and 9th weeks of feeding tended to have higher level, and the gap between the two groups was the biggest in the 6th week of feeding. In case of femur BMD, the effects of isoflavones supplementing appeared in the 6th weeks of feeding, and the effects was intensified in the 9th weeks of feeding to the extent that femur BMD in the IF group became significantly higher than that in the control group. As a result, the effects of isoflavones on BMD were different according to bone parts. The diet effects of the extracted isoflavones from soybean tend to come out earlier in spine that has more plentiful of trabecula bone than femur. Compared with cortical bone, trabecula bone has more active metabolism, so easily affected by metabolism (Pruitrt et al., 1992). Spine has more trabecula bone than cortical bone, while femur contains cortical bone more than 3 times than that of spine. That is why spine that has more trabecula bone is affected earlier than femur by isoflavones diet. Similar result was reported in the previous study(Choi & Cho, 2003) that provided soy isolate with rich isoflavones for growing female rats as well. It is also similar to the changing patterns in spine and femur between the previous study and this study.
The effects of soy isoflavones diet are also changed according to the period of diet intake. Previous epidemiological research showed that current diet amount of soy isoflavones affects spine BMD, but does not femoral neck and trochanter. On the other hand, the whole life diet amount of it significantly affects femoral neck, but not significantly affects spine, even though as diet amounts increase as the spine BMD high. However, since the study has reported that it does not affect trochanter, we can imply that same period of diet even its effects vary with regard to the bone parts (Rice, 1999).
Isoflavones' effect of protecting bone on pre-menopausal women who have enough estrogen varies to authors. Ho et al who has studied Hong Kong-resident Chinese women reported that diets of soybean for pre-menopausal women in the age of 30~40 brought about significant effect on spine BMD. Meanwhile, a massive size of epidemiological research on Chinese females in the age of 18~86 showed contrary results (Mei et al., 2001). The study reported that isoflavones diet affected BMD for post-menopausal women, but it had little to do with pre-menopausal women. Greendale et al. (2002) who studied Asian American women presented that genistein diets affected spine and femur BMD for pre-menopausal women, but no relevance to post-menopausal women.
Comparing with other study results might be difficult, since there are not much previous studies on growing female rats. But there are some relevant studies. Providing growing male rats with soy protein that contains isoflavones increased calcium efficiency on spine and femur BMD to a higher level than casein group (Jung, 1995). It is also reported that supplementing casein diet group of the growing male rats with isoflavones increases spine and femur BMD per weight level (Chae, 2002). It implies that soy protein or isoflavones should be beneficial to bone. A previous study (Choi & Cho, 2003) reported that isoflavones-rich soy protein should be beneficial to the formation of spine and femur BMD on growing female rats.
In summary, there was no significant difference in spine BMD between the IF group and the control group. But the IF group tended to have higher BMD than the control group in 6th week and 9th week of feeding and the striking difference could be shown in the 6th week of feeding. Spine BMD increase of the IF group in the week 3 to 6 after feeding was 37.4% more than that in the week 6 to 9 after feeding. In femur BMD, the effects of adding isoflavones appeared in the 6th week of feeding, and it became intensified in the 9th week of feeding to the extent that the BMD in the IF group was significantly higher than that of the control group (p<0.05).
In conclusion, isoflavone supplementation increased spine BMD per weight in the 6th week of feeding, and affected the increase of femur BMD in the 9th week. The result of the experiment implies that it affects positively the formation of spine and femur BMD of growing female rats. The study also can suggest that the effects of isoflavone on the pattern of BMD formation might differ from the parts of bones.
Go to : 
References
1. Adlercreutz H, Hamalainen E, Gorbach S, Goldin B. Dietary phyto-oestrogens and the menopause in Japan. Lancet. 1992; 339:1233. PMID: 1349965.

2. Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995; 333:276–282. PMID: 7596371.

3. Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996; 126:161–167. PMID: 8558297.

4. Brandi ML. Natural and synthetic isoflavone in the prevention and treatment of chronic diseases. Calcif Tissue Int. 1997; 61:S5–S8. PMID: 9263608.
5. Chae JH. The effect of isoflavones on bone mineral density and bone mineral content in growing male rats. 2002. Keimyung University of Korea;Mater's Thesis.
6. Choi MJ, Cho HJ. Effects of soy protein and isoflavones on bone mineral density in growing female rats. The Korean Journal of Nutrition. 2003; 36:359–367.
7. Eriksen EF, Colvard DS, Berg NJ. Evidence of estrogen receptors in normal human osteoblast like cells. Science. 1998; 241:84–86. PMID: 3388021.
8. Griffin MG, Kimble R, Hopfer W, Pacifici R. Dual energy x-ray absorptiometry of the rat: Accuracy, precision, and measurement of bone loss. J Bone Miner Res. 1993; 8:795–800. PMID: 8352062.
9. Greendale GA, Fitzgerald G, Huang MH, Sternfeld B, Gold E, Seeman T, Sherman S, Sowers M. Dietary soy isoflavones and bone mineral density : Results from the study of women's health across the nation. Am J Epidemiol. 2002; 155:746–754. PMID: 11943693.
10. Ishimi Y, Miyaura C, Ohmura M, Onoe Y, Sato T, Uchiyama Y, Ito M, Wang X, Suda T, Ikegami S. Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology. 1999; 140:1893–1900. PMID: 10098529.

11. Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002; 25:1709–1714. PMID: 12351466.

12. Jung SH. The effect of dietary protein source and sulfur amino acid content on bone metabolism in male rats. 1995. Keimyung University of Korea;Master's Thesis.
13. Kuiper GJM, Lemmen JG, Calsson B. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998; 139:4252–4263. PMID: 9751507.

14. Knight DC, Eden JA. A review of the clinical effects of phytoestrogens. Obstet Gynecol. 1996; 87:897–904. PMID: 8677131.
15. Lees CJ, Ginn TA. Soy protein isolate diet does not prevent increased cortical bone turnover in ovariectomized monkeys. Calcif Tissue Int. 1998; 62:557–558. PMID: 9576987.
16. Makela S, Davis VL, Tally WC. Dietary estrogens act through estrogen receptor-mediated processes and show no antiestrogenicity in cultured breast cancer cells. Environ Health Perspect. 1994; 102:572–578. PMID: 9679118.

17. Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001; 86:5217–5221. PMID: 11701680.

18. Miksicek RJ. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994; 49:153–160. PMID: 8031711.

19. Oursler MJ, Osdoby P, Pyfferoen J. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991; 88:6613–6617. PMID: 1907373.

20. Pruitt LA, Jackson RD, Bartels RL, Lehmard HJ. Weight-training effects on bone mineral density in early postmenopausal women. J Bone Miner Res. 1992; 7:179–185. PMID: 1570762.

21. Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents. J Nutr. 1993; 123:1939–1951. PMID: 8229312.
22. Rice MM. Soy consumption and bone mineral density in older Japanese American women in King county. 1999. Seattle, WA. USA: University of Washington;Ph.D Dissertation.
23. Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997; 127:263–269. PMID: 9039826.

24. Schonherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor-stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997; 339:353–361. PMID: 9056268.
25. Setchel KDR, Nechemias LZ, Cai J, heubi JE. Isoflavones content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998; 68:1453s–1461s. PMID: 9848516.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download