Abstract
Macrophages play a key role in iron metabolism by recycling iron through erythrophagocytosis. Ferroportin-1 (FPN1) is a transporter protein that is known to mediate iron export from macrophages. Since divalent metals often interact with iron metabolism, we examined if divalent metals could regulate the expression of FPN1 in macrophages. J774 macrophage cells were treated with copper, manganese, zinc, or cobalt at 10, 50, or 100 µM for 16 to 24 h. Then, FPN1 mRNA and protein levels were determined by quantitative real-time PCR and Western blot analyses, respectively. In addition, effects of divalent metals on FPN1 promoter activity were examined by luciferase reporter assays. Results showed that copper significantly increased FPN1 mRNA levels in a dose-dependent manner. The copper-induced expression of FPN1 mRNA was associated with a corresponding increase in FPN1 protein levels. Also, copper directly stimulated the activity of FPN1 promoter-driven reporter construct. In contrast, manganese and zinc had no effect on the FPN1 gene expression in J774 cells. Interestingly, cobalt treatment in J774 cells decreased FPN1 protein levels without affecting FPN1 mRNA levels. In conclusion, our study results demonstrate that divalent metals differentially regulate FPN1 expression in macrophages and indicate a potential interaction of divalent metals with the FPN1-mediated iron export in macrophages.
Iron homeostasis is achieved primarily through the regulation of its absorption and the conservation of body stores. While very little iron (1~2 mg/day) is newly absorbed from the diet in the duodenum, most of the iron required for erythropoiesis (~24 mg/day) is provided by recycling from senescent erythrocytes (Weiss, 2002). Macrophages of reticuloendothelial system, such as Kupffer cells in the liver and macrophages of spleen and bone marrow, play a key role in recycling iron through phagocytosis of erythrocytes and also serve as a major storage site for excess iron. Iron recycling by macrophages represents the largest pathway of intracellular iron export into the blood system (Ganz & Nemeth, 2006).
Ferroportin-1 (FPN1) is a protein with several transmembrane domains, which functions in the export of intracellular iron. Also known as MTP1 (metal transporter protein1), Ireg1 (iron regulated protein 1) and SLC40A1 (solute carrier family 40A1), FPN1 is highly expressed in tissues and cells associated with iron efflux such as duodenum, placenta, and macrophages of liver, spleen, and bone marrow (Abboud & Haile, 2000; Donovan et al., 2000; McKie et al., 2000). Stable overexpression of FPN1 in a mouse macrophage cell line resulted in a 70% increase in iron release after erythrophagocytosis (Knutson et al., 2005). In contrast, several mutations found in FPN1 gene in human were unequivocally associated with iron accumulation within the macrophages of liver and spleen and decreased serum iron concentration (Beutler, 2006; Pietrangelo, 2004). All of these evidences are consistent with the functional role of FPN1 as a central iron exporter.
Several factors are known to regulate FPN1 gene expression. For example, iron loading increased FPN1 in macrophages (Knutson et al., 2003; Yang et al., 2002a). The mRNA of FPN1 carries iron responsive element (IRE) in the 5'-untranslated region (UTR), thereby regulating FPN1 expression in a post-transcriptional way by the IRE/IRP (iron regulatory protein) system (McKie et al., 2000). However, the mRNA of FPN1 was also increased by iron loading in macrophages, which can not be explained by the IRE/IRP system. Thus, it is likely that several different regulatory mechanisms are present for FPN1 gene expression. Other factors that are known to regulate FPN1 expression include hypoxia (McKie et al., 2000) and inflammation (Liu et al., 2002; Ludwiczek et al., 2003; Yang et al., 2002b). Although divalent metals often interact with iron metabolism, whether these metals can influence FPN1 expression has not been thoroughly studied. In the present study, we evaluated the effects of various divalent metals such as copper, manganese, zinc and cobalt, on the regulation of FPN1 gene expression in macrophage cells.
J774 cells were obtained from the American Type Culture Collection (ATCC). Cells were maintained in α-minimum essential medium (Gibco Inc., USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin, and grown at 37℃ in a 5% CO2-95% air incubator with controlled humidity. Before metal treatments, cells were seeded in a 6-well plate and grown to 60~80% confluence. Stock solutions of CuSO4, MnCl2, ZnCl2, or CoCl2 were prepared at the concentration of 100 mM in PBS (pH 7.4) and sterilized by filtration with 0.2 µm membrane. All chemical reagents were purchased from Sigma (USA).
Total RNA was isolated using Trizol® reagent (Invitrogen, USA). Reverse transcription was carried out with 1 µg RNA samples using iScript™ cDNA synthesis kit (Bio-Rad, USA). The levels of FPN1 and 18S mRNA were determined by real-time PCR using iQ™ SYBR green supermix kit (Bio-Rad, USA) in a real-time PCR instrument. Primer sequences were FPN1: TTGCAG GAG TCA TTG CTG CTA and TGG AGT TCT GCA CAC CAT TGA T, 18S: CTG GCA CCA CAC CTT CTA and GGG CAC AGT TG GGT GAC (Xenotech, Korea). FPN1 gene cycle threshold (CT) numbers were normalized to 18S, and FPN1 mRNA content was calculated as the relative amount of FPN1 mRNA in treated cells compared to that of untreated controls (relative mRNA content=2-ΔΔCT).
FPN1 promoter/luciferase reporter gene construct (FPN1-Luc, a gift from Dr. Haile, University of Texas at San Antonio, USA) was used for this experiment. The FPN1-Luc plasmid consists of ~2.6 kb of FPN1 5' promoter region, all of the 5'-untranslated region (5'-UTR) including IRE, and firefly luciferase coding sequence. Empty vector that contains firefly luciferase coding sequence only was used as a negative control. For transient transfection, HeLa cells were seeded at 2.7×105 cells in a 6-well plate and grown to ~60% confluence. Either FPN1-Luc plasmid or empty vector was transiently transfected into HeLa cells using PolyFect® transfection reagent (QIAGEN, USA) according to manufacturer's manual. At 12-h after transfection, cells were washed and divided, and then treated with various metals for another 24 h. After metal treatments, cell lysates were prepared using 1x lysis buffer. Luciferase activity was measured with a luminometer using a luciferase reporter assay system (Promega, USA).
After metal treatment, cells were washed three times with PBS and lysed in a lysis buffer containing 4x Tris/SDS (pH 6.8), 5% b-mercaptoethanol, and protease inhibitor cocktails. After DNA shearing, protein concentrations of lysates were determined by RC DC Protein assay (Bio-Rad, USA). Fifteen micrograms of cell lysates were separated by SDS/polyacrylamide gel electrophoresis, and transferred to PVDF membranes. The membranes were stained with Ponceau-S solution to visualize and confirm that equal amounts of protein were transferred among wells. After rinsing Ponceau-S solution, the membranes were blocked with 5% skim milk in Tris-buffered saline (TBS) containing 0.01% Tween 20 for at least 1~2 hour. Blocked membrane was incubated with affinity purified anti-FPN1 antibody (1:100) overnight at 4℃. The membrane was then washed with TBS-T for 30 min and incubated with secondary anti-rabbit IgG peroxidase-linked antibody (Amersham, USA, 1:5000) in 2.5% skim milk/TBS-T for at least 1 hour at room temperature. Specific bands were visualized with an enhanced chemiluminescence system (ELPIS biotech, Korea). Intensities of the bands were quantified using a Gel Doc XR system (Bio-Rad, USA) equipped with Quantity one 1-D analysis software (Bio-Rad, USA).
All experiments were repeated three or four times on separate occasions. Statistical analysis was performed by using the SAS systems (ver. 9.1). Differences between groups were tested by ANOVA followed by Duncan's multiple comparison test. All data were expressed as mean ± SEM. P values less than 0.05 were considered significant.
To evaluate whether treatment of J774 macrophages with various metals affects the steady-state level of FPN1 mRNA, cells were treated with copper, manganese, zinc, or cobalt at indicated concentrations for 16 h and quantitative real-time RT-PCR analysis was performed. Metal treatments at above conditions did not change cell viability as determined by MTT assay. As shown in Fig. 1, copper treatment at 10 µM significantly increased FPN1 mRNA levels compared to untreated control. The increase of FPN1 mRNA by copper treatment was dose-dependent, and up to ~ 12-fold induction was observed with 100 µM copper treatment. In contrast, treatment with either manganese or zinc at the concentration range of 10 ~ 100 µM did not change FPN1 mRNA levels in J774 macrophages. Cobalt treatment at higher concentration (100 µM) increased FPN1 mRNA to some extent, but the increase did not reach to a statistical significance. In normal conditions, serum copper and zinc concentrations are approximately 25 µM and 15 µM, respectively (Hunt et al., 1998), and serum manganese and cobalt concentrations are much less. On the other hand, the serum and tissue levels of these metals can be greatly increased by environmental or occupational exposure to high doses of metals (Li et al., 2004) or under pathologic conditions (Cuthbert et al., 1995). Therefore, our study condition represents a wide range of situation from physiological condition to the status of metal intoxication.
To further examine whether divalent metals can directly modulate FPN1 promoter activity, we performed reporter assays using luciferase expression system. HeLa cells, which have no endogenous FPN1 expression, were transiently transfected with FPN1 promoter/luciferase reporter construct. Twelve hours after transfection, cells were treated with different metals and the luciferase activity was measured. Copper had a strong activating effect (~2.5 fold as compared to the control), while manganese, zinc or cobalt had minimal or no effect (Fig. 2).
To determine whether mRNA levels coincide with protein alteration, we performed western blot analyses. Our results showed that the up-regulation of FPN1 mRNA levels was concurrent with protein expression except for cobalt treatment. As shown in Fig. 3, copper greatly increased FPN1 protein levels, but manganese or zinc did not have any effect on FPN1 protein expression in J774 cells. Interestingly, different from mRNA levels, cobalt treatment in J774 macrophages significantly decreased FPN1 protein levels compared to untreated controls.
In the present study, we observed that, in addition to iron, the availability of copper regulates FPN1 gene expression in macrophage cells. Copper treatment in J774 macrophage cell line greatly induced the FPN1 mRNA with corresponding increase in FPN1 protein levels. Further, we demonstrated that copper treatment in mammalian cells transfected with FPN1 promoter-driven reporter constructs resulted in a strong induction of reporter activity, suggesting that copper directly stimulates FPN1 gene transcription. The interaction of an iron transporter with other divalent cations is also found in other transporter proteins. For example, divalent metal transporter 1 (DMT1) mediates the transport of many different metals including zinc, manganese, cobalt, copper as well as iron (Gunshin et al., 1997). DMT1 has a critical function as an iron transporter in the membrane of endosomes where transferrin-bound iron is released and exported to the cytosol, implicating that the presence of other divalent metals could affect iron release by DMT1. The regulation of FPN1 expression by copper observed in our study and in previous studies (Aigner et al., 2008; Andersen et al., 2007; Chung et al., 2004) suggests a key role of copper in iron release. Moreover, it is also possible that FPN1 may function as a common export protein for both iron and copper.
Unlike copper, treatment of J774 macrophages with manganese or zinc did not modulate FPN1 gene expression. Similar to our results, Wang et al. (2008) also reported that exposure to manganese in rat choroid plexus had no effect on the FPN1 mRNA and FPN1 protein levels. On the other hand, Yamaji et al. (2001) previously reported that zinc loading (100 µM) for 24 h significantly increased FPN1 mRNA in intestinal CaCo-2 cells, suggesting that zinc may regulate FPN1 gene expression in a cell-type specific manner. However, in the Yamaji's study (2001), changes in the FPN1 protein levels by zinc treatment were not measured and the zinc treatment (100 µM, 24 h) did not affect apical-to-basolateral iron transport in CaCo-2 cells. Therefore, it is likely that the induction of FPN1 mRNA by zinc in the intestinal cells has little physiological significance.
It is interesting to note that cobalt treatment at the concentration range of 10 to 100 µM in J774 cells did not affect FPN1 mRNA concentrations but significantly decreased FPN1 protein levels. It has been well documented that metals could regulate gene expression in a post-transcriptional manner. For example, iron regulates the expression of various genes, such as ferritin and transferin receptor (TfR), in a post-transcriptional manner through the IRE/IRP system. When cellular iron concentration is low, the binding activity of IRP to IRE is high. As a result, ferritin translation is blocked due to the presence of IRE in the 5'-UTR of ferritin mRNA. Conversely, TfR protein synthesis is increased by low iron. TfR mRNA contains multiple IREs in its 3'-UTR and the IRE-IRP complex stabilizes the TfR mRNA. As mentioned earlier, FPN1 mRNA contains IRE in its 5'-UTR. In fact, iron depletion by SIH (salicylaldehyde isonicotinoyl hydrazone, an iron-specific chelator) in macrophages resulted in a significant and dose-dependent decrease in FPN1 protein levels (Knutson et al., 2003). In the present study, the mechanism as to how cobalt affects FPN1 translation is unclear. But, one possibility is that cobalt treatment may indirectly decrease intracellular iron concentration, thereby lowering FPN1 protein levels.
The observation in which copper stimulates the FPN1 promoter activity provides strong evidence that copper-induced FPN1 expression is a transcription-dependent process. Copper has been associated with several different transcription factors in mammalian cells (González et al., 2008; Itoh et al., 2008; Mattie et al., 2004; Muller et al., 2007; Song et al., 2008). MTF-1 (metal response element (MRE)-binding transcription factor-1) is one of best known modes of action for the metal-regulated gene expression (Giedroc et al., 2001). Nevertheless, it is unlikely that MTF-1 is responsible for the copper-induced FPN1 expression, because zinc, more potent activator of MTF-1, did not affect FPN1 expression in macrophages. Recently, a DNA-response element called CABE (copper-responsive Atox1 binding element) has been shown to mediate copper-sensitive gene transcription (Muller et al., 2008). Whether the CABE is located in the FPN1 promoter region and mediates the copper-sensitive FPN1 transcription needs to be tested.
In summary, we demonstrated that FPN1 expression in macrophages is differentially regulated by various divalent metals. While manganese and zinc had no effect on FPN1 mRNA and protein levels, cobalt decreased FPN1 protein levels in a post-transcription manner. Further, copper significantly up-regulates FPN1 gene transcription with corresponding increase in FPN1 protein levels. Our data suggest a close interaction of copper and cobalt with FPN1-mediated iron release in macrophage cells.
Figures and Tables
 | Fig. 1
Effects of various divalent metals on FPN1 mRNA expression in J774 macrophages. J774 cells were treated with CuSO4, MnCl2, ZnCl2, or CoCl2 at indicated concentrations for 16 h. FPN1 mRNA and 18S mRNA concentrations were determined by quantitative real-time PCR analyses. Data are expressed as mean ± s.e. of three independent experiments. *p<0.05, compared to control (no metal treatment) |
 | Fig. 2
Effects of various divalent metals on FPN1 promoter activity. HeLa cells were transiently transfected with FPN1 promoter/luciferase (Luc) constructs and then treated with 100 µM CuSO4, MnCl2, ZnCl2, or CoCl2 for 24 h. HeLa cells transfected with an empty vector, which contains Luc coding sequence only without FPN1 promoter sequence, was used as a negative control. Luciferase activities of cell lysates were measured by luminometer. Data are expressed as mean ± s.e. of three or four independent experiments. *p<0.05, compared to control (no metal treatment) |
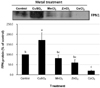 | Fig. 3
Effects of various divalent metals on FPN1 protein levels in J774 macrophages. J774 cells were treated with CuSO4, MnCl2, ZnCl2, or CoCl2 at 100 µM for 24 h. FPN1 protein concentrations were then determined by Western blot analyses. Upper panel: A representative blot from three independent experiments is shown. Lower panel: band density detected by chemiluminescence was quantified (QuantityOne imaging software, Bio-Rad). Values are mean ± s.e. of three independent experiments. Treatment not sharing the same superscript are significantly different from each other (p<0.05). |
References
1. Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000. 275:19906–19912.

2. Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008. 135:680–688.

3. Andersen HS, Gambling L, Holtrop G, McArdle HJ. Effect of dietary copper deficiency on iron metabolism in the pregnant rat. Br J Nutr. 2007. 97:239–246.

5. Chung J, Haile DJ, Wessling-Resnick M. Copper-induced ferroportin-1 expression in J774 macrophages is associated with increased iron efflux. Proc Natl Acad Sci U S A. 2004. 101:2700–2705.

6. Cuthbert JA. Wilson's disease: a new gene and an animal model for an old disease. J Investig Med. 1995. 43:323–336.
7. Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000. 403:776–781.

8. Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006. 1763:690–699.

9. Giedroc DP, Chen X, Apuy JL. Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxid Redox Signal. 2001. 3:577–596.

10. González M, Reyes-Jara A, Suazo M, Jo WJ, Vulpe C. Expression of copper-related genes in response to copper load. Am J Clin Nutr. 2008. 88:830S–834S.

11. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997. 388:482–488.

12. Hunt JR, Matthys LA, Johnson LK. Zinc absorption, mineral balance, and blood lipids in women consuming controlled lactoovovegetarian and omnivorous diets for 8 wk. Am J Clin Nutr. 1998. 67:421–430.

13. Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008. 283:9157–9167.

14. Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005. 102:1324–1328.

15. Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003. 102:4191–4197.

16. Li GJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004. 46:241–248.

17. Liu XB, Hill P, Haile DJ. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol Dis. 2002. 29:315–326.

18. Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003. 101:4148–4154.

19. Mattie MD, Freedman JH. Copper-inducible transcription: regulation by metal- and oxidative stress-responsive pathways. Am J Physiol Cell Physiol. 2004. 286:C293–C301.

20. McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000. 5:299–309.

21. Muller PA, Klomp LW. ATOX1: A novel copper-responsive transcription factor in mammals? Int J Biochem Cell Biol. 2008. BC-2807:1–4.

22. Muller PA, van Bakel H, van de Sluis B, Holstege F, Wijmenga C, Klomp LW. Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. J Biol Inorg Chem. 2007. 12:495–507.

24. Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, Kuo MT. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008. 74:705–713.

25. Wang X, Miller DS, Zheng W. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol. 2008. 230:167–174.

27. Yamaji S, Tennant J, Tandy S, Williams M, Singh Srai SK, Sharp P. Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells. FEBS Lett. 2001. 507:137–141.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download