Abstract
Atherosclerosis is characterized by a chronic inflammatory disease, and chemokines play an important role in both initiation and progression of atherosclerosis development. Leukotactin-1 (Lkn-1/CCL15), a new member of the human CC chemokine family, is a potent chemoattractant for leukocytes. Our previous study has demonstrated that Lkn-1/CCL15 plays a role in the initiation of atherosclerosis, however, little is currently known whether Lkn-1/CCL15 is associated with the progression of atherosclerosis. Matrix metalloproteinases (MMPs) in human coronary atherosclerotic lesions play a crucial role in the progression of atherosclerosis by altering the vulnerability of plaque rupture. In the present study, we examined whether Lkn-1/CCL15 modulates MMP-9 release, which is a prevalent form expressed by activated macrophages and foam cells. Human THP-1 monocytic cells and/or human peripheral blood monocytes (PBMC) were treated with phorbol myristate acetate to induce their differentiation into macrophages. Foam cells were prepared by the treatment of THP-1 macrophages with human oxidized LDL. The macrophages and foam cells were treated with Lkn-1/CCL15, and the levels of MMP-9 release were measured by Gelatin Zymography. Lkn-1/CCL15 significantly enhanced the levels of MMP-9 protein secretion from THP-1 monocytic cells-derived macrophages, human PBMC-derived macrophages, as well as macrophage-derived foam cell in a dose dependent manner. Our data suggest that the action of Lkn-1/CCL15 on macrophages and foam cells to release MMP-9 may contribute to plaque destabilization in the progression of atherosclerosis.
Early atherogenesis is characterized by the recruitment of circulating leukocytes (e.g., monocytes/macrophages and T-lymphocytes etc.) into artery wall, the formation of foam cells in the vessel intima, leading to the development of atherosclerotic lesions. The inflammatory responses in the atherosclerotic lesions play a crucial role to promote plaque rupture and thrombosis in the late progression of atherosclerosis, leading to myocardial infarction and strokes (Libby, 2002). The rupture-prone plaque is featured by a large lipid core and accumulation of inflammatory cells including macrophages and T-lymphocytes (Mann & Davies, 1996). A family of matrix metalloproteinases (MMPs), which are proteolytic enzymes secreted by the inflammatory cells, play a pivotal role in degrading extracellular matrix and plaque rupturing (Newby, 2005).
Chemokines, a superfamily of chemotactic cytokines, not only cause the recruitment of leukocytes into the arterial wall, but also influence atherosclerotic plaque stability by enhancing MMPs releases (Reape & Groot, 1999; Sheikine & Hansson, 2004), and thus play a crucial role in both the initiation and the progression of atherosclerosis. Leukotactin-1 (Lkn-1/CCL15), a new member of the human CC chemokine family, is a potent chemoattractant for leukocytes, and it is implicated in inflammatory pathologies including atherosclerosis. Our previous study and others have shown that the levels of Lkn-1/CCL15 expression are markedly up-regulated in macrophage-derived foam cells and in human atherosclerotic lesions (Lee et al., 2002; Yu et al., 2004), and moreover, the proatherogenic mediators such as oxidized lipoprotein and oxidative stress markedly upregulte Lkn-1/CCL15 gene expression and protein release from the foam cells (Yu et al., 2004), indicating Lkn-1/CCL15 plays a role in the initiation of atherogenesis. However, it remains unclear whether Lkn-1/CCL15 is associated with the progression of atherosclerosis.
In the present study, we investigated whether Lkn-1/CCL15 can enhance MMP-9 release from macrophages and/or foam cells, which influence plaque rupture. Our data demonstrated that Lkn-1/CCL15 enhanced MMP-9 protein release from human macrophages and macrophage-derived foam cells, indicating that Lkn-1/CCL15 may be associated with the vulnerability of plaque rupture in the late stage of atherosclerosis.
THP-1 cells were obtained from the Korean Cell Line Bank. THP-1 monocytes were maintained in the RPMI-1640 medium (Gibco). The cells were plated at a density of 5 × 105 cells/ml in the medium containing phorbol myristate acetate (PMA, Sigma) at 10-7 M for 48 h to induce their differentiation into macrophages.
Peripheral blood was collected from healthy donors. Peripheral blood mononuclear cells were separated on a Histopaque-1077 gradient. After washings twice with HBSS without Ca2+ and Mg2+, the PBMCs were then suspended in 10% FBS containing RPMI and incubated for 60 min at 37℃ to let the monocytes attach to the culture dish. The attached monocytes were harvested by vigorous pipetting and plated at a density of 5 × 105 cells/ml in the medium containing PMA at 10-7 M for 48 h to induce their differentiation into macrophages.
Human low density lipoprotein (LDL) (d = 1.006-1.063) was separated from freshly drawn normal plasma sample by sequential ultracentrifugation and extensively dialyzed at 4℃ against 0.15 mol/L NaCl and 0.01% EDTA. After EDTA was removed, LDL was subjected to oxidative modification by incubation with Cu2+ (5 µM CuSO4, 16 h at 37℃). Then Cu2+ was removed by extensive dialysis (Yu et al., 2004). The extent of modification was assessed by the determination of electrophoretic mobility on agarose gels. In agarose gel electrophoresis, oxidized LDL (oxLDL) moved two to three times faster than LDL did. OxLDL was stored at 4℃ and was used within 4 weeks of preparation.
To prepare macrophage-derived foam cells, human THP-1 cells were plated at a density of 5 × 105 cells/ml in the medium containing PMA at 10-7M for 48 h to induce their differentiation into macrophages, and then the cells were treated with human oxidized LDL (25 µg/ml) for 4 days (Yu et al., 2004).
The gelatinolytic activities of MMP-9 in the conditioning culture medium were assayed by electrophoresis on 10% polyacrylamide gels containing 1 mg/ml gelatin at 4℃. PAGE gels were run at 120 V, washed in 2.5% Triton X-100 for 1 h, and then incubated for 16 h at 37℃ in activation buffer (50 mM Tris-HCl, pH 7.5, 10 mM CaCl2). After staining with Coomassie blue (10% glacial acetic acid, 30% methanol and 1.5% Coomassie brilliant blue) for 2-3 h, the gel was destained with a solution of 10% glacial acetic acid, and 30% methanol without Coomassie blue for 1 h. White lysis zones indicating gelatin degradation were revealed by staining with Coomassie brilliant blue R-250.
The present study has demonstrated that Lkn-1/CCL15 enhances MMP-9 protein release by macrophages and macrophages-derived foam cells, indicating that Lkn-1/CCL15 may play a role in the plaque rupture of the atherosclerotic lesions.
Macrophages, which are abundant in ruptured atherosclerotic plaques, express multiple MMPs, weakening the plaque and making it rupture prone (Boyle, 2005). Among MMPs, MMP-9 (92-kDa gelatinase B), which is a prevalent form expressed by activated macrophages and foam cells, is found in human coronary atherosclerotic lesions (Galis & Khatri, 2002; Pasterkamp et al., 2000). We found that Lkn-1 significantly activated human THP-1 monocytes-derived macrophages and human PBMC-derived macrophages to release MMP-9 (Fig. 1A and 1B). It has been shown that the circulating levels of Lkn-1 are increased in plasma of atherosclerotic patients, and Lkn-1 stimulates endothelial cells to release intracellular adhesion molecule-1 (Yu et al., 2004). Since MMP-9 is required for monocyte infiltration through endothelium from blood, Lkn1/CCL15 action on macrophages to release MMP-9 may accelerate the recruitment of macrophages into the atherosclerotic lesions.
It has been shown that the expression levels of chemokine genes such as monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) are upregulated in human atherosclerotic lesions and they are associated with plaque rupture by activating endothelial cells and smooth muscle cells to release MMPs (Kodali et al., 2006; Reape et al., 1999; Sheikine et al., 2004; Shin et al., 2002). Atherosclerotic lesions consist of mainly macrophages and lipid-laden foam cells, and thus excessive production of MMP-9 by Lkn-1/CCL15 from macrophages and macrophage-derived foam cells in the lesions may accelerate the destabilization and rupture of the fibrous cap of the atherosclerotic plaque. Our data demonstrated that Lkn-1 markedly enhanced the production of MMP-9 from macrophage-derived foam cells (Fig. 2). We have previously shown that proatherogenic mediators enhance Lkn-1/CCL15 release from macrophages and macrophage-derived foam cells (Yu et al., 2004). Therefore, it can be postulated that macrophages and macrophage-derived foam cells in atherosclerotic lesions may enhance the local concentration of Lkn-1 in the lesions, and Lkn-1/CCL15 subsequently stimulate the macrophages/foam cells per se to secrete MMP-9, indicating that Lkn-1/CCL15 action on macrophages and foam cells to release MMP-9 may contribute to plaque destabilization in the progression of atherosclerosis.
In conclusion, Lkn-1/CCL15 enhanced MMP-9 protein secretion from THP-1 monocytic cells-derived macrophages, human PBMC-derived macrophages, and THP-1 macrophage-derived foam cells in a dose dependent manner. Considering the association of MMP-9 in plaque rupture, our findings suggest that Lkn-1/CCL15 could be associated with plaque destabilization in the progression of atherosclerosis. Lkn-1/CCL15 may be a useful target for the management of atherosclerosis. Further studies are needed to elucidate the inhibitory effect of phytochemicals or food factors on Lkn-1/CCL15 release from macrophages/foam cells.
Figures and Tables
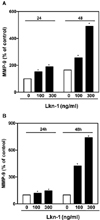 | Fig. 1
Effect of Lkn-1 on the production of MMP-9 protein from human THP-1 monocytic cells-derived macrophages, PBMC-derived macrophags, macrophages-derived foam cells. (A) Human THP-1 monocytic cells were plated at a density of 5 × 105 cells/ml in the medium containing PMA at 10-7M for 48 h to induce their differentiation into macrophages, and then the cells were treated with Lkn-1/CCL15 (100-300 ng/ml) for 24 h or 48 h. (B) Human peripheral blood monocytes were plated at a density of 5 × 105 cells/ml in the medium containing PMA at 10-7M for 48 h to induce their differentiation into macrophages, and then treated with Lkn-1/CCL15 (100-300 ng/ml) for 24 h or 48 h. The gelatinolytic activities of MMP-9 released into the conditioning culture medium were assayed by Gelatin Zymography described in the Materials and Methods. *P < 0.05, significantly different from the control without Lkn-1/CCL15 treatment. |
 | Fig. 2
Effect of Lkn-1 on the production of MMP-9 protein from macrophages-derived foam cells. Human THP-1 cells were plated at a density of 5 × 105 cells/ml in the medium containing PMA at 10-7M for 48 h to induce their differentiation into macrophages. THP-1 cells-derived macrophages were continuously treated with human oxidized LDL (25 µg/ml) for 4 days to prepare foam cells, and then the foam cells are treated with or without Lkn-1/CCL15 for 16 h. The gelatinolytic activities of MMP-9 released into the conditioning culture medium were assayed by Gelatin Zymography described in the Materials and Methods. *P < 0.05, significantly different from the control without Lkn-1/CCL15 treatment. |
References
1. Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005. 3:63–68.

2. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002. 90:251–262.
3. Kodali R, Hajjou M, Berman AB, Bansal MB, Zhang S, Pan JJ, Schecter AD. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc Res. 2006. 69:706–715.

4. Lee WH, Kim SH, Jeong EM, Choi YH, Kim DI, Lee BB, Cho YS, Kwon BS, Park JE. A novel chemokine, Leukotactin-1, induces chemotaxis, pro-atherogenic cytokines, and tissue factor expression in atherosclerosis. Atherosclerosis. 2002. 161:255–260.

6. Mann JM, Davies MJ. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996. 94:928–931.
7. Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005. 85:1–31.

8. Pasterkamp G, Schoneveld AH, Hijnen DJ, de Kleijn DP, Teepen H, van der Wal AC, Borst C. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000. 150:245–253.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download