Abstract
BACKGROUND/OBJECTIVES
One of the mechanisms considered to be prevalent in the development of Alzheimer's disease (AD) is hyper-stimulation of microglia. Black chokeberry (Aronia melanocapa L.) is widely used to treat diabetes and atherosclerosis, and is known to exert anti-oxidant and anti-inflammatory effects; however, its neuroprotective effects have not been elucidated thus far.
MATERIALS/METHODS
We undertook to assess the anti-inflammatory effect of the ethanolic extract of black chokeberry friut (BCE) in BV2 cells, and evaluate its neuroprotective effect in the lipopolysaccharide (LPS)-induced mouse model of AD.
RESULTS
Following stimulation of BV2 cells by LPS, exposure to BCE significantly reduced the generation of nitric oxide as well as mRNA levels of numerous inflammatory factors such as inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α). In addition, AD was induced in a mouse model by intraperitoneal injection of LPS (250 µg/kg), subsequent to which we investigated the neuroprotective effects of BCE (50 mg/kg) on brain damage. We observed that BCE significantly reduced tissue damage in the hippocampus by downregulating iNOS, COX-2, and TNF-α levels. We further identified the quinic acids in BCE using liquid chromatography-mass spectrometry (LCMS). Furthermore, we confirmed the neuroprotective effect of BCE and quinic acid on amyloid beta-induced cell death in rat hippocampal primary neurons.
Since aging is associated with increased incidences of brain diseases and disorders leading to economic and social burdens, the development of neuroprotective drugs is therefore an important and urgent issue [12]. Alzheimer's disease (AD) and dementia are neurodegenerative disorders characterized by a progressive loss of memory [3]. AD is etiologically complex, and has been hypothesized to result from factors such as accumulation of β-amyloid (Aβ) peptides, deposition of Aβ, hyper-phosphorylated tau, free radicals, and inflammatory reactions [4]. Although the precise causes of AD are still unknown, neuropathological changes in patients with AD typically include degeneration of the hippocampus following the Aβ peptide accumulation in the brain [56].
Molecules such as lipopolysaccharide (LPS) induce an excessive activation of microglia through overexpression of pro-inflammatory cytokines and inflammatory factors such as inducible nitric oxide (NO) synthase (iNOS), cyclooxygenase 2 (COX-2), and tumor necrosis factor alpha (TNF-α) [789]. Hyper-stimulated microglia play an important role in the neuropathogenesis and accumulation of Aβ by secreting cytokines [101112]. Experimental trials have shown that regulating the inflammatory process decreases the deposition of Aβ [13]. Natural products such as herbs, condiments, vegetables, and fruits may contain compounds that exert pharmacological and biological activities that inhibit the development or progression of AD [1415]. A functional food, black chokeberry (Arobia melanocarpa) has been shown to treat diabetes, and other studies have reported its beneficial effects on human health [161718]. However, the neuroprotective effects of black chokeberry have yet not been established.
This study therefore investigated the anti-inflammatory activity of black chokeberry fruit extract (BCE) on LPS-stimulated BV2 cells in vitro. In addition, we determined the neuroprotective effect of BCE in an LPS-induced mouse model of AD. Our findings demonstrate the biological activity of BCE against LPS-induced neurodegeneration, and our results inducate that BCE is a potential functional food for neuroprotection.
Materials for cell cultures were purchased from Gibco BRL (Carlsbad, CA, USA). Antibodies against iNOS, COX-2, and TNF-α were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The VECTASTAIN ABC Kit was purchased from Vector Laboratories (Burlingame, CA, USA). The LIVE/DEAD Cell Viability Assay Kit was purchased form Thermo Fisher (Grand Island, NY, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Dried black chokeberry fruits were purchased from Bandibulsanche Farm, Muju City, Republic of Korea. Two-hundred grams of the plant were blended and extracted for 24 h, using 1,000 mL of ehanol. The ethanol-soluble extracts were concentrated and evaporated at 60℃ under vacuum. The decocted solution was dissolved in 50 mL of sterile deionized water. The aqueous extract was lyophilized by freeze-drying at −60℃.
Mouse microglial BV2 cells were purchased from the Korean Cell Line Bank (Seoul, Korea) and cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS), at 37℃ and 5% CO2. Primray neuronal cells were isolated from prenatal Sprague Dawley (SD)-rats and grown in neurobasal medium containing 1% L-glutamine, 1% PS and B27 supplement, for 7 days at 37℃ and 5% CO2. Cell viability was assayed as in per protocol reported previously [19]. Morphological changes in the cells were observed, and images were captured using an inverted microscope connected to a digital camera (IX71; Olympus; Tokyo, Japan).
BV2 cells (5 × 104 cells/well in 96-well microplates) were co-treated with different concentrations of BCE (30 µg/mL to 1,000 µg/mL) in the absence or presence of LPS (500 ng/mL), and cultured for 48 h in DMEM supplemented with 10% FBS. To determine the total concentration of NO in the culture media, Griess reagent was added to 100 µL of supernatant at each treatment condition, and absorbance was measured at 520 nm using a microplate reader.
BV2 cells (5 × 104 cells/well in 96-well microplates) were treated with 300 µg/mL BCE in the absence or presence of LPS (500 ng/mL), and cultured for 24 h in DMEM supplemented with 10% FBS. Total RNA was isolated from cell lysates using the TRI reagent, following the manufacturer's protocol. mRNA expression was measured using a polymerase chain reaction (PCR) thermocycler with reverse transcriptase, as previously described [19]. Briefly, the first-strand cDNA was synthesized using SuperScript II Reverse Transcriptase following the manufacturer's protocol. The cDNA was then amplified using real-time PCR and thermocycler. Real-time PCR was performed under the following conditions: initial denaturation (10 min, 95℃), followed by 40 cycles of denaturation (30 sec, 95℃), annealing (30 sec, 60℃), and extension (30 sec, 72℃). The primers used were as follows: iNOS (5′-CAG GAG GAG AGA GAT CCG ATT-3′ and 5′-GCA TTA GCA TGG AAG CAA AGA-3′); COX-2 (5′-CTG GTC GGT TTG ATG CTA-3′ and 5′-CGA GTC GTT CTG CCA ATA-3′); IL-1β (5′-TAC CAG TTG GGG AAC TCT GC-3′ and 5′-TGG AAA AGC GGT TTG TCT TC-3′); TNF-α (5′-TGG GAG TAG ACA AGG TAC AAC-3′ and 5′-CAT CTT CTC AAA ATT CGA GTG-3′); GAPDH (5′-TGT CAT CAT ATC TGG CAG GTT-3′ and 5′-GGC CTT CCG TGT TCC TAC-3′).
Male ICR mice weighing 20–23 g were purchased from Orient Bio (Seongnam, Korea). The mice were individually housed with ad libitum access to water and food (AIN 93G formula). All mice were kept in a controlled environment (room temperature, 24 ± 2℃; humidity, 40 ± 2%; 12 h light/dark cycle). All experiments and animal care were conducted in accordance with the institutional guidelines of Dongguk University (IACUC-2014-005). The animals (6 weeks old; n = 30) were randomly divided into three groups. The untreated group (Control) received saline solution. The LPS-induced brain damage group (LPS) received a single intraperitoneal (i.p.) injection of 250 µg/kg LPS.
To evaluate the in vivo effect of BCE on LPS-induced neroinflammation, a non-toxic concentration was selected, as described previously [20]. The treatment group was orally administered BCE (50 mg/kg/day) in normal saline, followed by i.p. injection of 250 µg/kg LPS after 1 h, every day for 7 days.
Animals were sacrificed after 7 days and their brains were recovered, washed in ice-cold phosphate-buffered saline (PBS) and fixed in 2% formalin. Segments from each half were embedded in paraffin and serially sectioned into 5-µm slices. The prepared sections were cleared with xylene and hydrated with serial concentrations (70%, 80%, and 90%) of ethanol. Sections were stained with hematoxylin and eosin (H&E) or cresyl violet. Some sections were incubated overnight at 4℃, with primary antibodies for iNOS, COX-2, IL-1β and TNF-α. Immunohistochemistry was performed as previously report [21].
High-performance liquid chromatography (HPLC) analysis was carried out using a Sunfire C18 ODS 4.6 × 150 mm column (Waters Corporation, USA) connected to a photodiode array detector. The mobile phase was a mixture of 0.1% (v/v) acetonitrile and water containing 0.1% (v/v) formic acid, at a flow rate of 1 mL/min. A standard solution containing quinic acid (Sigma-Aldrich, MO, USA) was prepared by dissolving these compounds in distilled water (5 mg/mL). The solution was filtered through a 0.45-µm membrane filter, after which HPLC was performed.
The results are expressed as the mean ± standard deviation (SD) of at least three independent experiments (n ≥ 3). Differences between groups were determined using Student's t-test and one-way analysis of variance. Tukey's test was used for multiple comparisons (GraphPad Prism ver. 4.00 for Windows, La Jolla, CA, USA). P-values < 0.05 were considered statistically significant.
No changes were observed in the cell viability following treatment with BCE at concentrations of up to 2,000 µg/mL (Fig. 1A and 1B). Analysis of NO production in LPS-stimulated cells using Griess reagent assay showed that the NO levels were 21.87 ± 3.89 µM (P = 0.0001) following LPS treatment in control cells. The LPS-induced NO production decreased to 17.62 ± 3.31 µM (P = 0.0001) and 11.65 ± 3.89 µM (P = 0.0001) at concentrations of 300 µg/mL and 1,000 µg/mL of BCE, respectively (Fig. 1C).
At 300 µg/mL, BCE reduced the mRNA expression levels of LPS-induced inflammatory signals such as iNOS, COX-2, IL-1β, and TNF-α in LPS-stimulated BV2 cells to 81.71 ± 3.04% (P = 0.0001), 68.65 ± 14.17% (P = 0.0044), 100.19 ± 30.93% (P = 0.0006), and 97.83 ± 16.44% (P = 0.0001), respectively, as compared to LPS treated controls (Fig. 2A-D). BCE (30 µg/mL, 100 µg/mL, 300 µg/mL, 1,000 µg/mL, and 2,000 µg/mL) also significantly reduced the protein levels of LPS-induced inflammatory signals in a dose-dependent manner (Fig. 2D).
Fig. 3A shows representative images of the morphology of the dentate gyrus (DG) and cornu amonis (CA) regions in the hippocampus from LPS-induced mouse. Oral administration of BCE (50 mg/kg) significantly reduced the thin layer formation following LPS-induced brain damage. Analyses of protein expression levels of inflammatory factors and pro-inflammatory cytokines revealed that BCE significantly reduces the inflammatory signals in the LPS-stimulated CA1 region. Exposure to BCE also reduced the levels of iNOS, COX-2, IL-1β and TNF-α to 69.50 ± 5.22% (P = 0.0001), 53.90 ± 3.54% (P = 0.0001), 72.77 ± 11.09% (P = 0.0001) and 73.30 ± 7.03% (P = 0.0001), respectively, as compared to LPS only treatment (Fig. 3B).
Quinic acid was identified from among the mass constituents by its retention time, which was consistent with its molecular weight of 192.17 (Fig. 4). The amount of quinic acid in the BCE extract was found to be 0.023 mg/mg at the specified retention time (5 min).
The LIVE/DEAD Cell Assay Kit was used to determine the inhibitory effects of BCE and quinic acid on neuronal cell death. Fig. 5A shows the morphologies of cells treated with BCE or quinic acid, as well as untreated cells and cells treated with sodium dodecyl sulfate (SDS). The untreated cells did not display changes in morphology, while SDS (0.01%) significantly induced cell death. Treatment of neuronal cells with BCE (300 µg/mL and 1,000 µg/mL) or quinic acid (10 µM and 100 µM) did not significantly alter the cell viability when compared to the untreated group. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay determined the inhibitory effects of BCE and quinic acid on Aβ-induced primary cell death. Viability analysis of the primary neuronal cells revealed no change in cell viability following treatment with BCE at concentrations up to 300 µg/mL. Quinic acid concentrations up to 10 µM also had no effect on the viability of primary neurons (Fig. 5B and 5C). Aβ (20 µM) significantly decreased the primary neuronal cell viability, while BCE (100 µg/mL and 300 µg/mL) and quinic acid (10 µM) significantly reduced the Aβ-induced cell death (Fig. 5C and 5D).
Inflammation leads to progressive neuronal degenerative diseases by destroying the healthy tissue [4]. Gasparini et al. [22] reported that LPS influences Aβ deposition, and Lee et al. [23] demonstrated that LPS induces brain damage by causing an accumulation of Aβ. Studies by Yan et al. [24] presented that the anti-inflammatory drug ibuprofen significantly reduces the Aβ deposition in an AD mouse model. We therefore hypothesized that the active compounds from functional or medicinal foods could exert a neuroprotective effect. In addition to their nutritional value, functional foods contain active ingredients which induce biological activity; these components may reduce the risk of neuronal dysfunction [2526]. Some functional foods are shown to promote health and prevent disease. Black chokeberry (Arobia melanocarpa), which is regarded as a functional food, has been reported to have various biological functions [1718]. However, no studies on BCE and neuroprotection have been published to date. The first objective of our study was to investigate the effects of BCE on LPS-stimulated BV2 cells and LPS-induced damage in the mouse brain. We show that BCE was anti-inflammatory in vitro and in vivo, without affecting the viability of quiescent BV2 cells. These findings strongly support the idea that BCE confers protection against pathological neurodegeneration.
The excess production of NO following microglial activation contributes to the pathogeneses of neurodegenerative disorders. NO and iNOS, respectively, are known as a regulator and mediator that play key roles in the pathogenesis of AD [27]. In the present study, we examined the effects of BCE on LPS-induced NO release in BV2 cells. LPS-induced cells showed an increase in the expression levels of NO, whereas inflammatory factors (IL-1β, iNOS, COX-2, and TNF-α) decreased significantly following BCE treatment. We also demonstrated that BCE treatment modulates iNOS, COX-2, and TNF-α levels in the brain of LPS-stimulated mice. The DG and CA regions in the hippocampus are morphologically altered in AD. Neuronal cell death and overexpression of inflammatory signals such as iNOS, COX-2, and TNF-α are typical in these brain regions [28]. In our study, BCE significantly reduced the brain damage by regulating the expression levels of LPS-induced inflammation signals. These results suggest that BCE contains active molecules that regulate the inflammatory factors and pro-inflammatory cytokines.
Aβ is an important regulator of neuronal cell death and a key pathological molecule in AD. Prevention of pathological neuronal cell death still remains a major clinical challenge. This highlights the need for new therapeutic strategies [2930]. Our data indicates that the active compound in BCE potently regulated the Aβ-induced neuronal cell death. The second objective of our study was thus to identify the active molecules in BCE. Using gas chromatography mass spectrometry analysis, we identified (-)-quinic acid (10.77%) as the major chemical constituent in the ethanolic extract of black chokeberry (data not shown). We measured the level of quinic acid in the BCE extract using liquid chromatography-mass spectrometry (LC/MS). The present study revealed that quinic acid and BCE reduces the progression of Aβ-induced neuronal cell death. Aβ-induced neuronal death or neurodegeneration accelerates the progression of neuroinflammation. Pero et al. [31] demonstrated that there was no accumulation of quinic acid in the blood stream, but suggested that quinic acid could cross the brain blood barrier. Taken together, our results suggest that the quinic acid in BCE exerts its neuroprotective effects by reducing inflammation-induced neuronal damage.
In conclusion, our findings demonstrate that BCE reduces the expression of inflammatory factors and pro-inflammatory cytokines in the context of LPS-induced neuroinflammation. These results suggest that the quinic acid in BCE may have be protective against neurodegeneration. However, further studies are required to determine whether quinic acid is the major active compound. In addition, the concentrations of quinic acid leading to optimal health benefits need to be established. This is because we observed many peaks for other compounds in the LC/MS analysis of BCE.
Figures and Tables
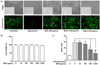 | Fig. 1Effects of black chokeberry extract on cell viability and production of nitric oxide (NO) in lipopolysaccharide-stimulated BV2 microglial cells.(A) The effects of BCE on cell viability were determined using the LIVE/DEAD Cell Viability Assay Kit. Morphological changes in BV2 cells were observed using microscopy (upper panel). The surviving cells were identified by their green fluorescence (bottom panel). (B) The effects of BCE on cell viability were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (n = 4). Cell viability is expressed as 100% in the untreated group. (C) Cells were co-treated with BCE and 500 ng/mL LPS for 48 hours. NO production was measured using the Griess reagent assay (n = 6). NO was quantified based on the sodium nitrate standard curve. Data are expressed as mean ± SD. *Significant difference when compared to LPS-only treatment (P < 0.05). BCE, black chokeberry extract; LPS, lipopolysaccharide.
|
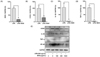 | Fig. 2Effects of black chokeberry extract on expression levels of inflammatory factors and pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 cells.(A-D) BV2 cells were seeded in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum for 24 hours, following which they were cultured in the absence or presence of LPS (500 ng/mL) and black chokeberry extract (BCE) (300 µg/mL) for 24 hours. (A-D) The relative levels of mRNA were calculated using 2−ΔΔCt values and normalized to that of GAPDH. (E) Proteins were separated and immunoblotted for protein expression analysis (COX-2, IL-1β, TNF-α, nuclear factor kappa-light-chain-enhancer of activated B cells, and GAPDH). *Significant difference when compared to LPS-only treatment (P < 0.05). LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase 2; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
|
 | Fig. 3Effects of black chokeberry extract on lipopolysaccharide-induced neuroinflammation.Representative photomicrographs of mouse brain cross-sections taken 7 days after an intraperitoneal injection of LPS. (A) Analysis of hippocampus morphology using hematoxylin and eosin staining. (B) Immunohistochemical staining for inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α) was performed in the cross-sections. Shading represents the intensity of brown color from iNOS, COX-2, IL-1β and TNF-α expression in the analyzed groups. Protein expression in the LPS group was considered to be 100%. Data are expressed as mean ± SD (n = 3). *Significant difference when compared to the LPS group (P < 0.05). BCE, black chokeberry extract; LPS, lipopolysaccharide; DG, dentate gyrus; CA1, cornu amonis 1; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase 2; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha.
|
 | Fig. 4LC-MS spectrum of black chokeberry extract and the chemical structure of quinic acid. MW: molecular weight. |
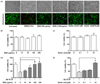 | Fig. 5Effects of black chokeberry extract and quinic acid on cell viability in Aβ-stimulated primary neuronal cells.(A) The effects of BCE and quinic acid on cell viability of primary neuronal cells were determined using the LIVE/DEAD Cell Assay Kit. The cell morphologies were compared to the untreated cell group. (B-D) Primary neuronal cells were cultured in medium containing BCE (10 µg/mL, 30 µg/mL, 100 µg/mL, or 300 µg/mL) and Aβ (20 µM) for 48 hours. The primary neuronal cells were also cultured in medium containing quinic acid (0.1 µM, 1 µM, or 10 µM) and Aβ (20 µM) for 48 hours. Cell viability were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell viability is expressed as 100% in the untreated group. Data are expressed as mean ± SD. *Significant difference when compared to the Aβ 25-35 group (P < 0.05). BCE, black chokeberry extract; Aβ 25-35, amyloid beta peptide 25-35.
|
Notes
References
1. Obisesan TO, Gillum RF, Johnson S, Umar N, Williams D, Bond V, Kwagyan J. Neuroprotection and neurodegeneration in Alzheimer's disease: role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in African Americans. Int J Alzheimers Dis. 2012; 2012:568382.

2. Ramírez BG, Blázquez C, Gómez del, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005; 25:1904–1913.

4. Choonara YE, Pillay V, du Toit LC, Modi G, Naidoo D, Ndesendo VM, Sibambo SR. Trends in the molecular pathogenesis and clinical therapeutics of common neurodegenerative disorders. Int J Mol Sci. 2009; 10:2510–2557.

5. Murphy MP, LeVine H 3rd. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010; 19:311–323.
6. LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007; 8:499–509.
7. Zotova E, Nicoll JA, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer's disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010; 2:1.

8. Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012; 32:11706–11715.

9. Choi SK, Park YS, Choi DK, Chang HI. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J Microbiol Biotechnol. 2008; 18:1990–1996.
10. Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer's disease. Microsc Res Tech. 2001; 54:59–70.

11. Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna). 2010; 117:949–960.

12. Nimmervoll B, White R, Yang JW, An S, Henn C, Sun JJ, Luhmann HJ. LPS-induced microglial secretion of TNFα increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb Cortex. 2013; 23:1742–1755.

13. Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med. 2015; 3:136.
14. Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006; 119:751–759.

15. Mao J, Huang S, Liu S, Feng XL, Yu M, Liu J, Sun YE, Chen G, Yu Y, Zhao J, Pei G. A herbal medicine for Alzheimer's disease and its active constituents promote neural progenitor proliferation. Aging Cell. 2015; 14:784–796.

16. Dragan S, Andrica F, Serban MC, Timar R. Polyphenols-rich natural products for treatment of diabetes. Curr Med Chem. 2015; 22:14–22.

17. Zapolska-Downar D, Bryk D, Małecki M, Hajdukiewicz K, Sitkiewicz D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur J Nutr. 2012; 51:563–572.

18. Jurikova T, Mlcek J, Skrovankova S, Sumczynski D, Sochor J, Hlavacova I, Snopek L, Orsavova J. Fruits of black chokeberry aronia melanocarpa in the prevention of chronic diseases. Molecules. 2017; 22:E944.

19. Lee KP, Kim JE, Park WH. Cytoprotective effect of rhamnetin on miconazole-induced H9c2 cell damage. Nutr Res Pract. 2015; 9:586–591.

20. Paulrayer A, Adithan A, Lee JH, Moon KH, Kim DG, Im SY, Kang CW, Kim NS, Kim JH. Aronia melanocarpa (black chokeberry) reduces ethanol-induced gastric damage via regulation of HSP-70, NF-κB, and MCP-1 signaling. Int J Mol Sci. 2017; 18:E1195.

21. Lee KP, Choi NH, Sudjarwo GW, Ahn SH, Park IS, Lee SR, Hong H. Protective effect of areca catechu leaf ethanol extract against ethanol-induced gastric ulcers in ICR mice. J Med Food. 2016; 19:127–132.

22. Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004; 88:337–348.

23. Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008; 5:37.

24. Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J Neurosci. 2003; 23:7504–7509.

25. Granado-Lorencio F, Hernández-Alvarez E. Functional foods and health effects: a nutritional biochemistry perspective. Curr Med Chem. 2016; 23:2929–2957.

26. Aronson JK. Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br J Clin Pharmacol. 2017; 83:8–19.

27. Asiimwe N, Yeo SG, Kim MS, Jung J, Jeong NY. Nitric oxide: exploring the contextual link with Alzheimer's disease. Oxid Med Cell Longev. 2016; 2016:7205747.

28. Danysz W, Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br J Pharmacol. 2012; 167:324–352.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download