1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.

2. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res Treat. 2016; 48:436–450.

3. Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003; 3:601–614.

4. Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2012; 71:75–83.

5. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988; 319:525–532.

6. Boyapati SM, Bostick RM, McGlynn KA, Fina MF, Roufail WM, Geisinger KR, Hebert JR, Coker A, Wargovich M. Folate intake, MTHFR C677T polymorphism, alcohol consumption, and risk for sporadic colorectal adenoma (United States). Cancer Causes Control. 2004; 15:493–501.

7. Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, Logan RF, Baron JA. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer. 2011; 129:192–203.

8. Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. 2011; 93:817–825.

9. Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005; 113:825–828.

10. Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Jacobs EJ, Leitzmann M, Mannisto S, Miller AB, Potter JD, Rohan TE, Schatzkin A, Speizer FE, Stevens VL, Stolzenberg-Solomon R, Terry P, Toniolo P, Weijenberg MP, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. 2010; 21:1919–1930.

11. Chuang SC, Rota M, Gunter MJ, Zeleniuch-Jacquotte A, Eussen SJ, Vollset SE, Ueland PM, Norat T, Ziegler RG, Vineis P. Quantifying the dose-response relationship between circulating folate concentrations and colorectal cancer in cohort studies: a meta-analysis based on a flexible meta-regression model. Am J Epidemiol. 2013; 178:1028–1037.

12. Martinez ME, Giovannucci E, Jiang R, Henning SM, Jacobs ET, Thompson P, Smith-Warner SA, Alberts DS. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer. 2006; 119:1440–1446.

13. Ibrahim EM, Zekri JM. Folic acid supplementation for the prevention of recurrence of colorectal adenomas: metaanalysis of interventional trials. Med Oncol. 2010; 27:915–918.

14. Ulrich CM, Potter JD. Folate and cancer--timing is everything. JAMA. 2007; 297:2408–2409.

15. Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994; 16:273–297.

16. Neugut AI, Jacobson JS, De Vivo I. Epidemiology of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1993; 2:159–176.
17. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007; 61:1435–1441.

18. Figueiredo JC, Levine AJ, Grau MV, Barry EL, Ueland PM, Ahnen DJ, Byers T, Bresalier RS, Summers RW, Bond J, McKeown-Eyssen GE, Sandler RS, Haile RW, Baron JA. Colorectal adenomas in a randomized folate trial: the role of baseline dietary and circulating folate levels. Cancer Epidemiol Biomarkers Prev. 2008; 17:2625–2631.

19. Song Y, Manson JE, Lee IM, Cook NR, Paul L, Selhub J, Giovannucci E, Zhang SM. Effect of combined folic acid, vitamin B(6), and vitamin B(12) on colorectal adenoma. J Natl Cancer Inst. 2012; 104:1562–1575.

20. Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, Hunter DJ, Giovannucci E. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. 2009; 90:1623–1631.

21. Gao QY, Chen HM, Chen YX, Wang YC, Wang ZH, Tang JT, Ge ZZ, Chen XY, Sheng JQ, Fang DC, Yu CG, Zheng P, Fang JY. Folic acid prevents the initial occurrence of sporadic colorectal adenoma in Chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res (Phila). 2013; 6:744–752.

22. Bird CL, Swendseid ME, Witte JS, Shikany JM, Hunt IF, Frankl HD, Lee ER, Longnecker MP, Haile RW. Red cell and plasma folate, folate consumption, and the risk of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1995; 4:709–714.
23. Levine AJ, Siegmund KD, Ervin CM, Diep A, Lee ER, Frankl HD, Haile RW. The methylenetetrahydrofolate reductase 677C-->T polymorphism and distal colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2000; 9:657–663.
24. Martinez ME, Henning SM, Alberts DS. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr. 2004; 79:691–697.

25. Marugame T, Tsuji E, Kiyohara C, Eguchi H, Oda T, Shinchi K, Kono S. Relation of plasma folate and methylenetetrahydrofolate reductase C677T polymorphism to colorectal adenomas. Int J Epidemiol. 2003; 32:64–66.

26. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.

27. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188.

28. Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558.

29. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006; 6:40.

30. Grizzle JE, Starmer CF, Koch GG. Analysis of categorical data by linear models. Biometrics. 1969; 25:489–504.

31. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

32. de Vogel S, Schneede J, Ueland PM, Vollset SE, Meyer K, Fredriksen A, Midttun O, Bjorge T, Kampman E, Bretthauer M, Hoff G. Biomarkers related to one-carbon metabolism as potential risk factors for distal colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2011; 20:1726–1735.

33. Fujimori S, Gudis K, Takahashi Y, Kotoyori M, Tatsuguchi A, Ohaki Y, Sakamoto C. Determination of the minimal essential serum folate concentration for reduced risk of colorectal adenoma. Clin Nutr. 2011; 30:653–658.

34. Ding H, Gao QY, Chen HM, Fang JY. People with low serum folate levels have higher risk of colorectal adenoma/advanced colorectal adenoma occurrence and recurrence in China. J Int Med Res. 2016; 44:767–778.

35. Le Marchand L, Wang H, Selhub J, Vogt TM, Yokochi L, Decker R. Association of plasma vitamin B6 with risk of colorectal adenoma in a multiethnic case-control study. Cancer Causes Control. 2011; 22:929–936.

36. Kim J, Cho YA, Kim DH, Lee BH, Hwang DY, Jeong J, Lee HJ, Matsuo K, Tajima K, Ahn YO. Dietary intake of folate and alcohol, MTHFR C677T polymorphism, and colorectal cancer risk in Korea. Am J Clin Nutr. 2012; 95:405–412.

37. Park JY, Vollset SE, Melse-Boonstra A, Chajes V, Ueland PM, Slimani N. Dietary intake and biological measurement of folate: a qualitative review of validation studies. Mol Nutr Food Res. 2013; 57:562–581.

38. Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997; 94:3290–3295.

39. Duthie SJ, Narayanan S, Blum S, Pirie L, Brand GM. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000; 37:245–251.

40. Choi SW, Kim YI, Weitzel JN, Mason JB. Folate depletion impairs DNA excision repair in the colon of the rat. Gut. 1998; 43:93–99.

41. Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER; Polyp Prevention Study G. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007; 297:2351–2359.

42. Eussen SJ, Vollset SE, Igland J, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, Overvad K, Tjonneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Weikert C, Pischon T, Linseisen J, Kaaks R, Trichopoulou A, Zilis D, Katsoulis M, Palli D, Berrino F, Vineis P, Tumino R, Panico S, Peeters PH, Bueno-de-Mesquita HB, van Duijnhoven FJ, Gram IT, Skeie G, Lund E, Gonzalez CA, Martinez C, Dorronsoro M, Ardanaz E, Navarro C, Rodriguez L, Van Guelpen B, Palmqvist R, Manjer J, Ericson U, Bingham S, Khaw KT, Norat T, Riboli E. Plasma folate, related genetic variants, and colorectal cancer risk in EPIC. Cancer Epidemiol Biomarkers Prev. 2010; 19:1328–1340.

43. Le Marchand L, White KK, Nomura AM, Wilkens LR, Selhub JS, Tiirikainen M, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009; 18:2195–2201.

44. Lee JE, Wei EK, Fuchs CS, Hunter DJ, Lee IM, Selhub J, Stampfer MJ, Willett WC, Ma J, Giovannucci E. Plasma folate, methylenetetrahydrofolate reductase (MTHFR), and colorectal cancer risk in three large nested case-control studies. Cancer Causes Control. 2012; 23:537–545.

45. Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S, Japan Public. Plasma folate and risk of colorectal cancer in a nested case-control study: the Japan Public Health Center-based prospective study. Cancer Causes Control. 2008; 19:67–74.

46. Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut. 2006; 55:1461–1466.

47. Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, Akhmedkhanov A, Zeleniuch-Jacquotte A, Riboli E. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer. 1999; 79:1917–1922.

48. Weinstein SJ, Albanes D, Selhub J, Graubard B, Lim U, Taylor PR, Virtamo J, Stolzenberg-Solomon R. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiol Biomarkers Prev. 2008; 17:3233–3240.

49. Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008; 134:29–38.

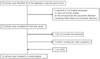








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download