Abstract
BACKGROUND/OBJECTIVES
Oxidative stress is closely related with inflammation and development of many diseases. Black soybean seed coat contains high amount of anthocyanins, which are well-known for free radical scavenging activities. This study investigated inflammatory response and action mechanism of black soybean anthocyanins with regard to antioxidant activity in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.
MATERIALS/METHODS
RAW 264.7 cells were treated with anthocyanins extracted from black soybean seed coats in a concentration range of 12.5 to 100 µg/mL. The production of reactive oxygen species (ROS), secretion of pro-inflammatory mediators and cytokines, and the signaling in the mitogen activated protein kinases (MAPKs) pathway were examined.
RESULTS
Black soybean anthocyanins significantly decreased LPS-stimulated production of ROS, inflammatory mediators such as nitric oxide (NO) and prostaglandin E2, and pro-inflammatory cytokines, including tumor necrosis factor α and interleukin-6, in a dose-dependent manner without cytotoxicity (P < 0.001). Black soybean anthocyanins downregulated the expression of inducible NO synthase and cyclooxygenase-2 in LPS-stimulated RAW 264.7 cells (P < 0.001). Moreover, black soybean anthocyanins inhibited LPS-induced phosphorylation of MAPKs, including extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 (P < 0.001).
Black soybean (Glycine max), a type of soybean with a black seed coat, has been widely used as a health promoting food and medicinal herb in Asia. In addition to various phytochemical compounds that are present in the common soybean, anthocyanins, which are compounds that impart colors to the epidermal layer of the black soybean, contribute to biological activities. Although the primary sources of anthocyanins in food are berries, grapes, and dark-colored vegetables [1], black soybean seed coat has been reported to contain an extremely high amount of anthocyanins, over 2,000 mg per 100 g [2]. Previous studies have shown that anthocyanins extracted from black soybeans exhibit various bioactivities such as antioxidative [3], anti-inflammatory [456], and anti-apoptotic effects [7]. However, the mechanisms underlying these effects have not been fully characterized.
Several reports indicate that chronic diseases associated with inflammation are characterized by excessive production of reactive oxygen species (ROS) [89]. Excessive ROS can lead to oxidative stress and elicit many pathological changes by damaging cellular lipids, proteins, and DNA. In addition, it has been demonstrated that ROS act as signaling molecules that provoke the production of inflammatory cytokines [10]. Although the cellular signaling pathways regulating inflammation are highly complicated, mitogen-activated protein kinases (MAPKs) and nuclear factor-κB (NF-κB) signaling are known to play important roles in the signal transduction for the expression of inflammatory mediators [11]. MAPKs include extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. The activation of MAPKs is important to the production of inflammatory mediators via control of NF-κB activation [12]. It has been reported that anthocyanins have antioxidant properties through oxygen radical scavenging activity [13]. All of these studies suggest that anthocyanins may act by mechanisms linking ROS and inflammation.
Macrophages play a key role in inflammatory responses by producing a variety of inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), and by producing pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, and IL-6 [14]. Lipopolysaccharide (LPS)-stimulated RAW 264.7 cells are extensively used as a model for evaluating the potency of anti-inflammatory agents and exploring their mechanism of action because LPS is a potent inducer of inflammatory responses. Although anti-inflammatory activities of black soybean anthocyanins have been reported in previous studies [456], there have been no published reports on inflammatory response in LPS-stimulated macrophages. In this study, we examined the antioxidant activity of black soybean anthocyanins and later investigated the effect on inflammatory mediators and MAPKs activation in LPS-stimulated RAW 264.7 cells, enabling us to determine the inflammatory response mediated by anti-oxidant activity.
The RAW 264.7 macrophage cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Fetal bovine serum, RPMI 1640 medium, and penicillin-streptomycin were purchased from GIBCO (Grand Island, NY, USA). A 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay kit was purchased from Promega (Madison, WI, USA). The enzyme immunoassay (EIA) kits for PGE2, TNFα, and IL-6 were obtained from R&D systems (Minneapolis, MN, USA). Antibodies specific for inducible NO synthase (iNOS) and cyclooxygenase (COX)-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other antibodies used were purchased from Cell Signaling Technology (Beverly, MA, USA). 2,7-Dichlorofluorescein diacetate (DCFH-DA) was purchased from Molecular Probes (Eugene, OR, USA). LPS and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Black soybean anthocyanin extract was offered from Rural Development Administration (RDA), Republic of Korea. The anthocyanins were extracted from Cheongja3 black soybean seed coat. The extraction method and identification of major compounds were described in a study by Lee [15]. High performance liquid chromatography (HPLC) was performed for quantification and identification of anthocyanins in the extract using Dionex Ultimate 3000 series (Dionex Softron GmbH, Germering, Germany) dual low pressure ternary gradient pump and Ultimate 3000 series photodiode array detector. Anthocyanins represented 85.3% of the extract. The composition of anthocyanins, determined by the peak area ratio on HPLC, consisted of cyanidine-3-O-glucoside (68.3%), delphinidin-3-O-glucoside (25.2%), and petunidin-3-O-glucoside (6.5%).
RAW 264.7 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. The cells were cultured at 37℃ in a humidified 5% CO2 incubator. A black soybean anthocyanin stock solution (100 mg/mL) was made by dissolving the extract in DMSO. This stock solution was diluted to appropriate concentrations with DMEM before use. DMSO (0.1% v/v) was used as the control. Cell viability was determined by MTS assay kit. In brief, the cells were seeded in 96-well plates at a density of 5 × 104 cells/well and various concentrations of black soybean anthocyanins (6.25, 12.5, 25, 50, 100, and 200 µg/mL) were added after 24 h of culture. After 24 h of incubation, the formazan concentration was measured at 490 nm using a microplate reader (ELx808, Biotek, Winooski, VT, USA) to determine.
Intracellular ROS production was measured using DCFH-DA. DCFH-DA is hydrolyzed to DCFH by deacetylase within the cells and oxidized by a variety of intracellular ROS to DCF, a highly fluorescent compound [16]. The cells were seeded in 6-well plates (1 × 106 cells/well) and incubated for 24 h. The cells were pre-treated with the indicated concentrations of black soybean anthocyanins for 1 h and were stimulated with LPS (1 µg/mL) for 24 h. Next, the cells were washed with serum free medium and treated with 20 µM DCFH-DA for 30 min at 37℃ in a CO2 incubator. The DCF level was measured using a flow cytometer (Cytomics FC500, Beckman Coulter, Brea, CA, USA).
Nitrite is a stable end product of nitric oxide generated by activated macrophages. We measured the nitrite accumulation in the cell culture supernatant with Griess method as previously described [17]. RAW 264.7 cells (5 × 105 cells/well) were seeded in 12-well plates and incubated for 24 h. The cells were treated with the indicated concentrations of black soybean anthocyanins for 1 h before exposure to LPS (1 µg/mL). After incubation for 24 h, the concentration of nitrite in the culture supernatant was determined by measuring absorbance at 540 nm.
The concentrations of PGE2, TNFα, and IL-6 in the cell culture supernatant were determined using EIA kits according to the manufacturer's instructions. RAW 264.7 cells were seeded in 6-well plates (1 × 106 cells/well) for the PGE2 assay and in 24-well plates (1 × 105 cells/well) for the TNFα and IL-6 assays. After 24 h of culture, cells were treated with the indicated concentrations of black soybean anthocyanins for 1 h before LPS (1 µg/mL) treatment. The supernatants were collected after 24 h of incubation and used for the assays.
Western blot assay was performed as described previously [17]. Briefly, RAW 264.7 cells (1 × 106 cells/well) were seeded in 6-well plates and cultured for 24 h, and then treated with the indicated concentrations of black soybean anthocyanins for 1 h before exposure to LPS (1 µg/mL) for the indicated times. Cells were collected and lysed in a cold RIPA buffer (pH 7.4). After centrifugation at 10,000 × g for 10 min at 4℃, aliquots of lysates with an equal amount of protein (20-30 ug) were separated by 10% SDS-PAGE and then transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The membrane was incubated overnight with specific primary antibodies (iNOS, COX-2, ERK, phospho-ERK, JNK, phospho-JNK, p38, and phospho-p38) at 4℃ and further incubated with secondary horseradish peroxidase-conjugated antibodies for 1 h at room temperature. The immunoactive proteins were detected with an ECL system (Ab frontier, Seoul, Korea), and quantified with a FluorChem densitometer and the Image J Program (National Institute of Health, Bethesda, MD, USA).
Data were analyzed using SAS software (Version 9.2, SAS Institute, Inc., Cary, NC, USA). The results were presented as means ± standard deviation (SD) and all experiments were performed at least three times. A one-way ANOVA followed by Tukey's post-hoc test was used to determine the differences among the treatment groups. P values of less than 0.05 were considered to be significant.
The cytotoxicity of black soybean anthocyanins to RAW 264.7 cells was measured by MTS assay. Cell viability was not significantly affected by black soybean anthocyanins given at concentrations up to 100 µg/mL (Fig. 1). Therefore, cells were treated with black soybean anthocyanins in a concentration range of up to 100 µg/mL for the following experiments.
To investigate whether black soybean anthocyanins influence ROS level, ROS was measured based on the DCF fluorescence intensity in LPS-stimulated RAW 264.7 cells. As shown in Fig. 2, stimulation with LPS resulted in a marked increase in DCF fluorescence intensity and treatment with black soybean anthocyanins reduced the increase in fluorescence intensity dose-dependently (P < 0.001). ROS level was decreased by 34.1% and 60.5% with 25 and 100 µg/mL of black soybean anthocyanins, respectively.
NO production increased significantly in RAW 264.7 cells stimulated with LPS. However, treatment with black soybean anthocyanins significantly inhibited LPS-stimulated NO production (P < 0.001). The nitrite concentration decreased by 27.9% and 57.2% with black soybean anthocyanins treatment at the levels of 25 and 100 µg/mL compared with the LPS-induced control cells, respectively (Fig. 3A). Treatment of cells with black soybean anthocyanins also inhibited LPS-induced PGE2 production in a dose-dependent manner (P < 0.001). PGE2 production was decreased by 43.6%. 71.8%, and 87.8% with 12.5, 50, and 100 µg/mL of black soybean anthocyanins, respectively (Fig. 3B).
We determined whether the inhibitory effects of black soybean anthocyanins on the production of NO and PGE2 were related to changes in the expression of iNOS and COX-2 proteins. The expression of iNOS and COX-2 proteins was increased in response to LPS, while treatment with black soybean anthocyanins inhibited these increases (P < 0.001, Fig. 3C and 3D). When regression analysis was performed, the production of NO and PGE2 was positively correlated with the expression of iNOS and COX-2 proteins, respectively (r2 = 0.5270, P < 0.001 for between NO and iNOS, r2 = 0.6204, P < 0.001 for between PGE2 and COX-2).
To investigate the effect of black soybean anthocyanins on the production of pro-inflammatory cytokines, levels of TNFα and IL-6 were measured. Stimulation with LPS resulted in a significant increase in production of TNFα and IL-6 in RAW 264.7 cells. However, these increases were significantly inhibited by treatment with black soybean anthocyanins in a dose-dependent manner (P < 0.001, Fig. 4). The levels of TNFα were decreased by 21.0%, 69.1%, and 73.2% after treatment with 12.5, 50, and 100 µg/mL of black soybean anthocyanins compared with the LPS-stimulated cells, respectively. Similarly, pre-treatment of the cells with black soybean anthocyanins decreased IL-6 production by 22.2%, 48.1%, and 75.8% when 12.5, 50, and 100 µg/mL black soybean anthocyanins were used in the culture, respectively.
After 30 min of stimulation with LPS, the effect of pre-treated black soybean anthocyanins on the activation of MAPKs was determined by measuring the levels of both phosphorylated and total protein for ERK, JNK, and p38. As shown in Fig. 5, LPS treatment significantly increased the level of the phosphorylated form of ERK, JNK, and p38. However, black soybean anthocyanins inhibited the LPS-induced phosphorylation of these three MAPKs in a dose dependent manner (P < 0.001). Listed from strongest to weakest, the inhibitory effect was evident for p38, JNK, and ERK. The ratios of phosphorylated p38 to total p38 were decreased by 58.1%, 21.8%, and 12.7% after treatment with 12.5, 50, and 100 µg/mL of black soybean anthocyanins compared with the LPS-stimulated cells, respectively. JNK activation decreased by 74.0%, 57.2%, and 39.6% when 12.5, 50, and 100 µg/mL of black soybean anthocyanins were used to pre-treat the cultures, respectively. The inhibitory effect on ERK activation was modest compared with that observed for p38 and JNK activation.
Oxidative stress is closely related with inflammation and development of several chronic diseases [10]. Although previous studies have reported the anti-inflammatory activities of black soybean anthocyanins in several models [456], little is known about the mechanisms that underlie the anti-inflammatory effect of black soybean anthocyanins with regard to antioxidant activity. We found that black soybean anthocyanins reduced the production of ROS and inflammatory mediators and decreased activity in MAPKs signaling pathway in LPS-stimulated RAW 264.7 cells.
In this study, stimulation with LPS resulted in an accumulation of intracellular peroxide. Black soybean anthocyanins significantly attenuated the LPS-induced increase in intracellular ROS. Oxidative stress results from an imbalance between ROS production and the antioxidant defense system. Anthocyanins are known as potential natural scavengers of ROS [13]. Black bean seed coat contains high amounts of anthocyanins, including cyanidin-3-glucoside, delphinidin-3-glucoside, and petunidin-3-glucoside. Cyanidin-3-glucoside, which comprised 68.3% of black soybean anthocyanins used in our study, was reported to have the highest radical quenching activity among anthocyanins [13]. In addition, anthocyanins were reported to induce the expression of antioxidant defense enzymes such as superoxide dismutase and glutathione-related enzymes [1819]. These findings suggest that the antioxidant activity of black soybean anthocyanin might be due to upregulation of the cellular defense system in addition to direct scavenging of ROS.
Inflammation is an important host response to infection and injury. Inflammatory responses are associated with increased production of NO, PGE2, and inflammatory cytokines, such as IL-6 and TNFα [14]. However, uncontrolled and excessive production of these inflammatory mediators causes a detrimental effect to the host. Therefore, these inflammatory mediators are regarded as targets for anti-inflammatory agents. NO is a major well-known pro-inflammatory mediator and contributes to the inflammatory cascade by increasing vascular permeability and the extravasation of fluids and proteins at inflammatory sites [20]. PGE2 acts as a mediator of inflammation by promoting local vasodilation and local attraction and activation of inflammatory cells at the early stages of inflammation [21]. Additionally, several pro-inflammatory cytokines, such as TNFα and IL-6, are known to play important roles in the induction of inflammation. TNFα is involved in the production of numerous cytokines and acute phase proteins, contributing to many pathophysiological processes [22]. IL-6 is an important mediator of fever and the acute phase response, which initiates the innate immune system in response to infection [23]. It has been reported that black soybean anthocyanins have protective activity in several inflammation models [456]. However, there were limited data on the effect of black soybean anthocyanins on inflammatory mediators. In this study, we demonstrated the anti-inflammatory effects of black soybean anthocyanins in LPS-stimulated RAW 264.7 cells as treatment with black soybean anthocyanins inhibited the production of NO, PGE2, TNFα, and IL-6 in dose dependent manners without a cytotoxic effect. These results are consistent with the results from a previous study using BV2 microglial cells [24]. The anti-inflammatory effect of black soybean anthocyanins is at least partly attributed to cyanidin-3-glucoside, considering that exposure of macrophages to cyanidin-3-glucoside from black rice resulted in a reduction of LPS-induced TNFα and IL-1β [25].
NO and PGE2 are synthesized by iNOS and COX-2, respectively, in response to inflammatory stimuli. We examined the effect of black soybean anthocyanins on the protein expression of iNOS and COX-2 to investigate the underlying mechanism of its inhibitory effect on NO and PGE2 production. We found that the protein expression of iNOS and COX-2 was reduced by treatment with black soybean anthocyanins and positively correlated with the production of NO and PGE2, respectively. This finding indicated that black soybean anthocyanins inhibit the production of NO and PGE2 in LPS-induced RAW 264.7 cells by suppressing the expression of protein enzymes responsible for the production of them. The dose-dependent suppression of NO concentration can be attributed to the direct free radical scavenging activity of black soybean anthocyanins in addition to the inhibition of iNOS protein expression. Also, it has been shown that low level of NO can enhance expression of iNOS in some cells [26], which may be related to the relative weak correlation between NO concentration and iNOS protein expression in the range of 50-100 µg/mL.
Furthermore, the production levels of inflammatory mediators are interrelated with one another. Previous studies reported that the expression of iNOS is stimulated by pro-inflammatory cytokines, such as IL-1β and TNFα, and TNFα-stimulated IL-6 production is a prerequisite for increased production of NO [2728]. In this study, black soybean anthocyanins exerted a dose-dependent reduction in LPS-induced production of IL-6 and TNFα, which could contribute to the decreased production of NO through inhibition of iNOS expression.
Considering that increased ROS production can activate diverse downstream signaling pathways [2930], ROS may act as signal-transducing molecules in the signaling and regulation of biological responses elicited by inflammation. We hypothesized that black soybean anthocyanins alleviate LPS-induced inflammatory response by modulating ROS signaling pathways through their antioxidant actions. In this study, treatment with black soybean anthocyanins reduced LPS-induced ROS production and inhibited the activation of all three MAPKs in RAW 264.7 macrophages. The activation of MAPKs is involved in LPS-induced expression of inflammatory mediators and the activation of NFκB, a transcriptional factor that regulates a number of genes important for inflammation [31]. Anthocyanin extract from black bean seed coat has been reported to inhibit activation of NF-κB in H.pylori-infected human gastric epithelial cells [5] and in ultraviolet B (UVB)-exposed human keratinocyte HaCaT cells [32]. MAPKs are regulated by phosphorylation and dephosphorylation. ROS has been shown to inhibit activation of MAPK phosphatase (MKP)-1 leading to a sustained JNK activation, which contributes to TNFα-induced cell death [33]. Blocking of ROS production by antioxidants inhibited LPS-induced MAPKs phosphorylation and inflammatory cytokine production in mouse embryonic fibroblasts [34]. These reports suggest that ROS function as an upstream regulator of MAPKs. On the other hand, it was reported that the overexpression of MKP-1 or specific inhibitors of p38 and JNK inhibited ROS production in LPS-activated BV-2 cells [35]. This paradox could be due to the complexity of involved networks as well as different metabolic mechanisms according to cell types. Emre et al. [36] proposed a signal amplification loop, in which LPS stimulation of macrophage quickly down-regulated uncoupling protein 2 (UCP2) through the JNK and p38 pathways. This down-regulation was shown to increase ROS production in order to potentiate MAPK activation for inflammatory response [36]. It seems that the observed suppression of ROS production by specific inhibitors of p38 and JNK in the previous study [35] could be ascribed to sustained UCP2 expression and inhibition of the LPS-stimulated ROS signal amplification loop. Therefore, it is hard to establish cause and effect relationship or identify upstream and downstream events due to complex link between ROS production and signaling pathways. Nevertheless, it is likely that due to its antioxidant property, black soybean anthocyanins decrease ROS production, thereby reduce MAPK activation and inflammatory cytokine production in this study. This study has the limitation that direct mechanism of anti-inflammatory effects by black soybean anthocyanins was not delineated. Further research on kinetic effect of black soybean anthocyanins with ROS scavenger or specific inhibitors of signaling pathway in oxidative stress will be required to elucidate direct action of black soybean anthocyanins in signaling pathways.
In conclusion, this study demonstrates that black soybean anthocyanins suppress LPS-stimulated production of NO and pro-inflammatory mediators in RAW 264.7 cells by inhibiting intracellular generation of ROS and activation of MAPKs. Black soybean anthocyanins might have potential as a therapeutic agent for the treatment of various inflammatory diseases.
Figures and Tables
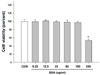 | Fig. 1Effect of black soybean anthocyanins (BSA) on the viability of RAW 264.7 cells.Cells were treated with the indicated concentrations of BSA for 24 h, and the cell viability was measured with a MTS assay. The data are presented as the means ± SD (n = 4). *P < 0.001 was used to indicate a significant difference compared with the control by ANOVA followed by student's t-test.
|
 | Fig. 2Effect of black soybean anthocyanins (BSA) on LPS-induced ROS production in RAW 264.7 macrophages.The cells were treated with the indicated concentrations of BSA for 1 h and later stimulated with LPS (1 µg/mL) for 24 h. Intracellular peroxide was measured by labeling with DCFH-DA for 30 min, and then analyzing the fluorescent intensity using a flow cytometer. The data are presented as the means ± SD from three independent experiments. Mean values without a common letter are significantly different as determined by a one-way ANOVA followed by Tukey's test (P < 0.001). LPS, lipopolysaccharide; CON, control; ROS, reactive oxygen species; DCFH-DA, 2,7-Dichlorofluorescein diacetate.
|
 | Fig. 3Effect of black soybean anthocyanins (BSA) on NO/PGE2 production and iNOS/COX-2 expression in RAW 264.7 cells.The cells were treated with the indicated concentrations of BSA for 1 h and later stimulated with LPS (1 µg/mL) for 24 h. The levels of nitrite (A) and PGE2 (B) were measured in the culture media using the Griess reagent and an EIA kit, respectively. Western blot analysis was performed to determine the protein levels of iNOS (C) and COX-2 (D). The data are presented as the means ± SD from three independent experiments. Mean values without a common letter are significantly different as determined by a one-way ANOVA followed by Tukey's test (P < 0.001). LPS, lipopolysaccharide; CON, control; NO, nitric oxide; PGE2, prostaglandin E2; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2.
|
 | Fig. 4Effect of black soybean anthocyanins (BSA) on the production of pro-inflammatory cytokines in RAW 264.7 cells.The cells were treated with the indicated concentrations of BSA for 1 h and later stimulated with LPS (1 µg/mL) for 24 h. The protein levels of TNFα (A) and IL-6 (B) were measured in the culture media by EIA. The data are presented as the means ± SD from three independent experiments. Mean values without a common letter are significantly different as determined by a one-way ANOVA followed by Tukey's test (P < 0.001). LPS, lipopolysaccharide; CON, control; TNFα, tumor necrosis factor alpha; IL, interleukin.
|
 | Fig. 5Effect of black soybean anthocyanins on the activation of MAPKs in RAW 264.7 cells.The cells were treated with the indicated concentrations of black soybean anthocyanins for 1 h and later stimulated with LPS (1 µg/mL) for 30 min. Western blot analysis was performed to determine the protein levels of JNK (A), ERK (B) and p38 (C). The data are presented as the means ± SD from three independent experiments. Mean values without a common letter are significantly different as determined by a one-way ANOVA followed by Tukey's test (P < 0.01). LPS, lipopolysaccharide; CON, control; JNK, c-Jun N-terminal kinase; p-JNK, phospho-c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p-ERK, phospho-extracellular signal-regulated kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
|
Notes
References
1. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006; 54:4069–4075.

2. Choung MG, Baek IY, Kang ST, Han WY, Shin DC, Moon HP, Kang KH. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J Agric Food Chem. 2001; 49:5848–5851.

3. Zhang RF, Zhang FX, Zhang MW, Wei ZC, Yang CY, Zhang Y, Tang XJ, Deng YY, Chi JW. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J Agric Food Chem. 2011; 59:5935–5944.

4. Kim HJ, Xu L, Chang KC, Shin SC, Chung JI, Kang D, Kim SH, Hur JA, Choi TH, Kim S, Choi J. Anti-inflammatory effects of anthocyanins from black soybean seed coat on the keratinocytes and ischemiareperfusion injury in rat skin flaps. Microsurgery. 2012; 32:563–570.

5. Kim JM, Kim KM, Park EH, Seo JH, Song JY, Shin SC, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiol Immunol. 2013; 57:366–373.

6. Sohn DW, Bae WJ, Kim HS, Kim SW, Kim SW. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology. 2014; 84:1112–1116.

7. Mok JW, Chang DJ, Joo CK. Antiapoptotic effects of anthocyanin from the seed coat of black soybean against oxidative damage of human lens epithelial cell induced by H2O2. Curr Eye Res. 2014; 39:1090–1098.

8. Drake IM, Mapstone NP, Schorah CJ, White KL, Chalmers DM, Dixon MF, Axon AT. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H pylori eradication. Gut. 1998; 42:768–771.

9. Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014; 5:352.

10. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49:1603–1616.

11. Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, Oh KW, Han DC, Kwon BM, Hong JT. Inhibitory effect of 2‘-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem Pharmacol. 2005; 69:791–799.

12. Suh SJ, Chung TW, Son MJ, Kim SH, Moon TC, Son KH, Kim HP, Chang HW, Kim CH. The naturally occurring biflavonoid, ochnaflavone, inhibits LPS-induced iNOS expression, which is mediated by ERK1/2 via NF-kappaB regulation, in RAW264.7 cells. Arch Biochem Biophys. 2006; 447:136–146.

13. Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997; 45:304–309.

14. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286.

15. Lee JH, Kang NS, Shin SO, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha TJ. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009; 112:226–231.

16. Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983; 130:1910–1917.
17. Kim MH, Kim JN, Han SN, Kim HK. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. 2015; 37:228–235.

18. Hashimoto N, Noda T, Kim SJ, Yamauchi H, Takigawa S, Matsuura-Endo C, Suzuki T, Han KH, Fukushima M. Colored potato extracts induce superoxide dismutase-2 mRNA via ERK1/2 pathway in HepG2 cells. Plant Foods Hum Nutr. 2010; 65:266–270.

19. Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007; 55:9427–9435.

20. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54:469–487.
23. Singh PP, Goyal A. Interleukin-6: a potent biomarker of mycobacterial infection. Springerplus. 2013; 2:686.

24. Jeong JW, Lee WS, Shin SC, Kim GY, Choi BT, Choi YH. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int J Mol Sci. 2013; 14:1502–1515.

25. Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010; 10:959–966.

26. Luss H, DiSilvio M, Litton AL, Molina y Vedia L, Nussler AK, Billiar TR. Inhibition of nitric oxide synthesis enhances the expression of inducible nitric oxide synthase mRNA and protein in a model of chronic liver inflammation. Biochem Biophys Res Commun. 1994; 204:635–640.

27. Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-κB. Glia. 2003; 41:152–160.

28. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007; 27:2576–2581.

29. Racz B, Hanto K, Tapodi A, Solti I, Kalman N, Jakus P, Kovacs K, Debreceni B, Gallyas F Jr, Sumegi B. Regulation of MKP-1 expression and MAPK activation by PARP-1 in oxidative stress: a new mechanism for the cytoplasmic effect of PARP-1 activation. Free Radic Biol Med. 2010; 49:1978–1988.

30. Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005; 6:587–592.

31. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.

32. Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J Agric Food Chem. 2008; 56:8969–8974.

33. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005; 120:649–661.

34. Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med. 2011; 208:519–533.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download