Abstract
BACKGROUND/OBJECTIVES
Complications of diabetes, such as cardiovascular disease, are associated with increased mortality among type 2 diabetes mellitus patients. Homocysteine has been recently identified as a predictor of cardiovascular disease-related complications in diabetes. We investigated whether or not supplementation with folic acid tablets can lower homocysteine levels and improve parameters related with vascular complications.
SUBJECTS/METHODS
We conducted a non-randomized 8-week trial involving postmenopausal diabetic women (n = 25) supplemented with 800 µg of folic acid (400 µg twice a day) daily. Subjects' serum levels of folate, homocysteine, and vitamin B12 were measured, along with vascular function and brachial-ankle pulse wave velocity.
RESULTS
Folic acid supplementation significantly increased serum folate levels (P < 0.0001), reduced homocysteine levels (P < 0.0001), and increased vitamin B12 levels (P = 0.0063). There were significant decreases in low-density lipoprotein cholesterol levels as well as the ratios of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol and total cholesterol to high-density lipoprotein cholesterol. Brachial-ankle pulse wave velocities were not altered by supplementation. Changes in serum vitamin B12 after folic acid supplementation were negatively correlated with changes in brachial-ankle pulse wave velocity.
Type 2 diabetes mellitus (T2DM) is a risk factor for cardiovascular disease (CVD), and T2DM patients are at increased risk of morbidity and mortality from CVD [1]. The presence of T2DM also increases the relative risk (RR) of developing CVD in women (RR = 8.5) compared to men (RR = 3.2) [1]. Major CVD risk factors that have been identified in T2DM patients include arterial stiffening [2], endothelial dysfunction [3], hyperglycemia [4], and elevated glycated haemoglobin (HbA1c) concentrations [5]. The UK Prospective Diabetes Study has showed that tight control of blood pressure and blood glucose concentration among T2DM patients does not reduce risk of myocardial infarction and peripheral vascular disease [6].
High levels of homocysteine (Hcys) have been identified as a risk factor for CVD in T2DM patients [7]. High concentrations of Hcys are associated with increased low-density lipoprotein oxidation [8] and endothelial dysfunction [9]. High concentrations of Hcys can worsen T2DM by inducing reversible dysfunction of β-islet cells [10] and inhibiting secretion of insulin [11]. Levels of plasma Hcys increase in women after menopause, and post-menopausal diabetic women are consequently at significantly increased risk of CVD [1213].
Folic acid supplementation may improve the cardiovascular health of post-menopausal women with diabetes. In particular, folic acid supplementation has been shown to reduce Hcys levels and improve vascular health in different study populations. Studies have also shown that folic acid supplementation can slow progress of atherosclerosis [14], improve plasma folate levels, and reduce plasma Hcys levels [15]. Folic acid supplementation also improves vascular parameters in post-menopausal women [16]. Further, studies on healthy, post-menopausal Egyptian [13], Italian [17], and Pakistani [18] women have shown that folic acid supplementation improves Hcys levels. Similarly, several studies have reported that folic acid supplementation can improve Hcys levels among T2DM patients [19] and post-menopausal women at high risk of developing CVD [20]. However, to our knowledge, no such studies have examined the post-menopausal diabetic female population, particularly in South-east Asia.
From the 1990s to 2013 in Korea, there has been an increase in T2DM incidences from 7.2% to 11% [21]. Though the CVD mortality rate has decreased in Korea over the past decade, the disease burden remains high [22]. Thus, this study aimed to investigate whether or not folic acid supplementation can lower Hcys levels and improve vascular parameters in post-menopausal diabetic Korean women.
The participants in this study included female patients with T2DM who visited Huh's Diabetes Clinic in Seoul, Korea. Between September 2005 and February 2011, 854 patients visited the clinic [21]. For this study, post-menopausal women aged 56-74 years with amenorrhea for over 24 months and serum Hcys ≥ 10 µmol/L were considered. The top 20th percentile of serum Hcys among our participants was 10 µmol/L. Thus, serum Hcys ≥ 10 µmol/L was used as the inclusion criterion. The participants received treatment for T2DM; other medications and diet therapy remained unchanged during the study period. Exclusion criteria included receiving hormone replacement therapy or having consumed folic acid supplements in the past 12 weeks. During the consultation time, study participants were advised to not make any changes in their lifestyle behaviors such as dietary habits. The 31 participants in this study consumed 800 µg of folic acid daily (400 µg twice a day) for 8 weeks. In this study, no side effects were reported. Overall, 81% (n = 25) of participants had > 70% compliance and were included in this statistical analysis. The remaining quantity of folic acid tablets was used to estimate the compliance rate of the study participants. Thus, the total number of participants eligible for the final analysis was 25. All participants volunteered for the study, and informed consent was obtained. The research protocol was approved by the Institutional Review Board of Ewha Womans University, Seoul, Korea (IRB No. 100-3).
Information on age, education, employment status, smoking habits, alcohol consumption, exercise pattern, duration of T2DM, family history of diabetes, and hypertensive and hyperlipidemia status was collected through questionnaires. The standing heights of the participants were measured using a stadiometer (Seca Inc., Hamburg, Germany). The body weights of the participants were measured using InBody 4.0 (Biospace Co., Ltd, Seoul, Korea). Body mass index (BMI; kg/m2) was calculated using height and weight measurements.
In this study, 800 µg of folic acid daily (400 µg twice a day) for 8 weeks was administered to the study participants. A folic acid supplementation study among the Netherlands population, including post-menopausal women aged 50-70 years administered 800 µg of folic acid to the study participants for 3 years [23]. The Korean dietary reference intake (DRI) recommends 1,000 µg per day as the tolerable upper intake levels for folic acid intake. Supplementation with 800 µg of folic acid was carried out based on compliance with Korean DRI recommendations and the existing literature.
The mini dietary assessment (MDA) questionnaire was used to assess dietary habits among the study subjects before and after supplementation. The MDA questionnaire was in Korean and consisted of a total of 10 components. The questionnaire collected data about the participants' dietary habits, including daily intake of dairy products, fish and meat consumption, vegetable and fruit intake, as well as consumption of fatty and salty foods and confectionaries. In the questionnaire, four components consisted of encouraged behaviors while three components consisted of restricted behaviors. The answer choices were “always,” “generally,” and “seldom.” For the four encouraged components, the answer choice “always” received a score of 5, “generally” a score of 3, and “seldom” a score of 1. The score points were reversed for restricted components. The total scores from each question were calculated to obtain the dietary habit score, and the total possible score was 50.
Blood samples were drawn from the participants after a 12-hour overnight fast. The samples were collected in tubes containing Ethylenediaminetetraacetic acid, followed by centrifugation at 3,000 rpm for 20 min at 4℃ (Hanil Science Industrial Co., Ltd, Seoul, Korea). Serum folate, vitamin B12, and Hcys were measured using chemiluminescence immunoassays (ADVIA Centaur XP, Siemens Healthcare Diagnostic, USA). Triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and fasting blood sugar (FBS) were measured using an autoanalyzer (Cobas Mira Roche, Hoffmann-La Roche Ltd., Basel, Switzerland). The following equation, developed by Friedewald [24] and Lauer [25], was used to calculate low-density lipoprotein cholesterol (LDL-C) levels.
Measurements of HbA1c were taken using an autoanalyzer (HLD-723 G7, Tosoh Corporation, Tokyo, Japan).
After allowing the participants to rest for 10 min, systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements were taken in a sitting position using an automatic blood-pressure monitor (Biospace Co., Seoul, Korea). The average of the SBP and DBP measured from the left and right arms was used for the analyses.
The moving pulse wave velocity between two different points on the ankle and brachial artery was measured using an automated analyzer (VP-1000; Colin Co. Ltd., Komaki, Japan). Brachial-ankle pulse wave velocity (baPWV) was calculated further using the moving pulse wave velocity between the ankle and brachial artery and the following equation based on transmission distance, which was calculated based on the subject's height [26].
The average value of the left and right baPWV was used in the analyses. Blood pressures of the ankle and brachial arteries were measured using a bidirectional Doppler (BiDop ES-100V, Koven Technology, St. Louis, USA) and mercury sphygmomanometer [27]. The ankle SBP value was divided by the brachial SBP value to obtain the ankle brachial index (ABI) for the respective leg [27]. The average ABI values from the left and right legs were used for the analysis. Radial pulse was measured for 15 seconds in a seated position after resting for 5 minutes. The value was then multiplied by 4 to obtain pulse rate (beats/min). The average pulse rate of the left and right hands was used for the analysis.
General characteristics of the subjects were expressed as means ± SD (continuous data) or as numbers with percentages (categorical data). The paired t-test was used to determine differences in mean anthropometric and clinical characteristics before and after folic acid supplementation. For further analysis, data were categorized using the following cut-off values: SBP ≥ 130 mmHg, DBP ≥ 85 mmHg, FBS ≥ 100 mg/dL, TG ≥ 150 mg/dL, TC ≥ 240 mg/dL, HDL-C < 50 mg/dL, LDL-C ≥ 70 mg/dL, LDL-C/HDL-C > 2 [28], TC/HDL-C > 3 [28], TG/HDL-C > 4 [29], HbA1c > 6%, Hcys ≥ 15 µmol/L, folate < 7.0 ng/mL [30], vitamin B12 < 300 pg/mL, ABI < 1.0 [31], baPWV ≥ 1,566 cm/s [32], and pulse rate > 60 beats/min. McNemar's test was used to analyze changes in anthropometric and clinical characteristics before and after folate supplementation. Pearson's correlation test was performed to evaluate the relationship between changes in Hcys, folate, vitamin B12, and other variables after supplementation. All statistical analyses were performed using the SAS statistical package (SAS 9.3, SAS Institute Inc., Cary, NC, USA). The level of significance set for the study was P < 0.05.
The mean age of the participants was 66.0 ± 5.5 years (Table 1). The mean BMI of the participants before supplementation was 24.3 ± 3.5 kg/m2. The mean duration of diabetic status was 13.4 ± 5.4 years, and 55.0% of participants had a family history of diabetes. Among the participants, 95.5% were non-smokers.
There was no significant difference (P = 0.8647) in mean MDA scores before (34.3 ± 4.7) and after supplementation (33.8 ± 5.3) (Table 2). Further, there were no significant changes in weight and BMI after supplementation.
There was a significant increase in serum folate levels, from 14.1 ± 6.6 ng/mL to 44.4 ± 9.6 ng/mL, after supplementation (P < 0.0001) (Table 2). Serum vitamin B12 levels also significantly increased by 16.2% from 528.2 ± 176.7 pg/mL to 613.7 ± 176.0 pg/mL after supplementation (P = 0.0027). There was a 22.2% decrease in serum Hcys levels after supplementation from 15.3 ± 3.8 µmol/L to 11.9 ± 2.6 µmol/L (P < 0.0001). The baseline values for vitamin B12, TG, TC, HDL-C, TG/HDL-C ratio, and DBP were within healthy cut-off values. After folic acid supplementation, mean serum Hcys was within the healthy range of < 15 µmol/L. There were no significant changes in the vascular parameters baPWV and pulse rate after folic acid supplementation. Folic acid supplementation reduced LDL-C level (P = 0.0018) as well as LDL-C/HDL-C and TC/HDL-C ratios. No significant changes were observed in other clinical parameters, including BMI, SBP, FBS, HbA1c, TG, and HDL-C.
Clinical characteristics before and after folic acid supplementation were compared using cut-off values. Clinical characteristics for which significant results were observed are presented in Table 3. The number of participants with Hcys ≥ 15 µmol/L decreased from 9 (36.0%) to 3 (12.0%) after folic acid supplementation.
Correlation analysis for changes in clinical characteristics before and after supplementation found significant changes only in serum Hcys, folate, serum vitamin B12, and baPWV levels (data not shown). An inverse correlation (r = -0.4876, P = 0.0134) was observed between changes in serum Hcys and folate levels after supplementation (Fig. 1). An inverse correlation between changes in serum vitamin B12 and baPWV levels (r = -0.4153, P = 0.0390) (Fig. 2) was also observed after 8 weeks of folic acid supplementation.
The effects of folic acid supplementation on Hcys levels and vascular parameters in post-menopausal Korean women with T2DM were investigated. Daily supplementation with 800 µg of folic acid for 8 weeks significantly improved serum Hcys, folate, and vitamin B12 levels as well as lipid parameters, including LDL-C level as well as LDL-C/HDL-C and TC/HDL-C ratios. Folic acid supplementation did not improve the vascular parameter baPWV.
Several other studies have reported increased folate and vitamin B12 levels and reduced Hcys levels after folic acid supplementation. Folic acid supplementation in post-menopausal women or T2DM or coronary heart disease patients has been associated with increased folate levels [151619]. Hcys levels were shown to be reduced in healthy Egyptian and Italian post-menopausal women after folic acid supplementation [1317]. Folic acid is an essential vitamin cofactor required for the remethylation of Hcys to methionine in the Hcys metabolism pathway [3334]. The serum vitamin B12 level in our subjects increased significantly by 16.2% after folic acid supplementation. Another folic acid supplementation study on healthy postmenopausal women reported a non-significant 2% increase in serum vitamin B12 levels [17]. Vitamin B12 is another important vitamin cofactor necessary for the remethylation of Hcys [34]. High Hcys levels trap vitamin B12, which becomes bound to methionine synthase as methyl-cobalamin [35]. The increase in remethylation of Hcys to methionine after folic acid supplementation releases vitamin B12 from methionine synthase, thereby increasing levels of vitamin B12. Folic acid supplementation was thus able to increase remethylation of Hcys and reduce accumulation of Hcys by increasing serum folate and vitamin B12 levels. In our study, mean serum Hcys level was within the healthy range of < 15 µmol/L after supplementation. Changes in biochemical parameters before and after folic acid supplementation were also analyzed using appropriate cut-off values. Before folic acid supplementation, 36% of our study participants had hyperhomocysteinemia, which was reduced to 12% by the end of the study. Studies have shown that an increase in Hcys levels to 15-25 µmol/L can be attributed to dietary deficiencies in folic acid and vitamins B6 and B12 [3336].
Folic acid supplementation significantly reduced LDL-C levels as well as LDL-C/HDL-C and TC/HDL-C ratios in this supplementation trial. Further, analysis using cut-off values reported significant reduction of LDL-C/HDL-C ratio (> 2 value) in participants. In our study, 70.8% of the population had hyperlipidemia. The current literature has not stratified subjects based on medication status for analysis [37]. In our study, there were no significant changes in weight, BMI, and dietary habit score in MDA after 8 weeks of folic acid supplementation. However, there were 7.5% and 14.3% reductions in LDL-C levels and LDL-C/HDL-C ratios, respectively, after supplementation. A study on healthy post-menopausal Italian women similarly observed a 27.5% reduction in LDL-C/HDL-C ratio after folic acid supplementation [17]. An experimental study on cystathionine-β-synthase-/-/apolipoprotein E-/- mice reported an association between higher Hcys levels as well as decreased HDL-C and increased TC and non-HDL-C levels [38]. High Hcys levels were also shown to reduce the enzymatic activities of two HDL-metabolism related enzymes, hepatic thiolase and lecithin-cholesterol acyltransferase [39]. Increased levels of Hcys were shown to induce mitochondrial stress, leading to an increase in the expression of sterol regulatory element-binding protein increasing expression of LDL receptors and LDL-C uptake [40]. The improvements in lipid parameters might be due to the reduction of serum Hcys levels caused by folic acid supplementation. Vitamin B12 deficiency in patients with T2DM taking metformin, a standard drug treatment for T2DM, has been well established. Circulating vitamin B12 level was shown to be reduced by 25% when using metformin, which is inversely correlated with lipid profile parameters such as TG level and TC/HDL-C ratio [41]. The role of vitamin B12 as a coenzyme for fatty acid oxidation is affected by vitamin B12 deficiency, resulting in lipogenesis and accumulation of lipids [41]. The 16.2% increase in serum vitamin B12 level in our study after supplementation could have caused reduction of LDL-C level through increased fatty acid oxidation.
Pulse wave velocity (PWV), a measure of arterial stiffness [42] and distensibility [32], is regarded as an indicator of vascular health [42]. In the present study, we observed no change in the vascular parameter baPWV after folic acid supplementation. Another folic acid supplementation study on T2DM patients also reported no significant improvement in carotid-radial and carotid-femoral PWV after 4 weeks [42]. Several studies performed flow-mediated dilation to investigate improvement of vascular parameters after folic acid supplementation. Folic acid supplementation was shown to improve flow-mediated dilation in post-menopausal women [16] and coronary heart disease patients [15]. Lowered Hcys levels might improve endothelial function, as increased plasma Hcys levels are associated with endothelial dysfunction through activation of coagulation factors, changes in adhesive properties of the endothelial surface [43], and increased oxidation of LDL-C [44].
In our study, we observed a significant inverse relationship between changes in vitamin B12 level and baPWV. Other researchers reported a significant inverse relationship between vitamin B12 and PWV in T2DM patients [42]. The mechanism responsible for the increase in vitamin B12 and decrease in baPWV is unclear but might be related to asymmetrical dimethylarginine (ADMA). ADMA has been identified as a competitive endogenous inhibitor of nitric oxide synthase, a necessary enzyme for nitric oxide synthesis [4546]. Increased ADMA levels have been associated with increased PWV [47] and endothelial dysfunction [46]. Methylation of L-arginine-containing proteins produces ADMA [46]. The methyl source for this methylation process, which eventually produces Hcys, is S-adenosylmethionine [46]. Vitamin B12 plays an important role in the remethylation of Hcys to S-adenosylmethionine. Further, vitamin B12 supplementation studies have reported that vitamin B12 reduces levels of ADMA [4548]. This reduction of ADMA caused by an increase in vitamin B12 levels might have led to the inverse association between changes in vitamin B12 level and baPWV.
This folic acid supplementation study has some limitations. This was a non-randomized trial, and the small sample size of the study (n = 25) might account for the lack of statistically significant changes in variables such as baPWV. Furthermore, the non-randomized study design and small sample size makes it difficult to generalize the study results. Further, the lack of a placebo control prevents any determination on whether or not the results were due to supplementation alone. Lack of dietary information, such as 24-hour dietary recall data, makes it difficult to understand how individual dietary habits may have interacted with supplementation. In our study, there was a lack of information on medications taken by the participants, which made determination of the possible interactions between supplementation, medications, and other dietary nutrients challenging. Nevertheless, this is the first study to investigate the effects of folic acid supplementation on Hcys levels and vascular parameters among post-menopausal Korean women with T2DM.
In conclusion, folic acid supplementation might be beneficial for reducing Hcys levels and decreasing lipid parameters in post-menopausal women with T2DM. We observed that folic acid supplementation reduced serum Hcys levels, increased serum folate and vitamin B12 levels, and lowered lipid parameters such as LDL-C levels as well as LDL-C/HDL-C and TC/HDL-C ratios. In our study, changes in serum vitamin B12 levels as a result of supplementation were inversely correlated with changes in baPWV. In the future, further analysis of folic acid supplementation, Hcys levels, and vascular parameters among post-menopausal Korean women using a larger sample size and randomization is necessary. Placebo control or collection of 24-hour food recall data will help differentiate between the effects of daily food intake and changes due to folate supplementation.
Figures and Tables
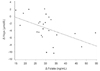 | Fig. 1Correlations between changes in serum Hcys and folate in post-menopausal diabetic women before and after folic acid supplementation (r = -0.4876, P-value = 0.0134).Hcys: homocysteine
|
 | Fig. 2Correlations between changes in serum vitamin B12 and baPWV levels (r = -0.4153, P-value = 0.0390) in post-menopausal diabetic women before and after folic acid supplementation.baPWV: brachial-ankle pulse wave velocity
|
Table 2
Anthropometric and biochemical parameters before and after supplementation with folic acid in post-menopausal diabetic women

MDA, mini dietary assessment; BMI, body mass index; Hcys, homocysteine; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBS, fasting blood sugar; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; baPWV, brachial-ankle pulse wave velocity; ABI, ankle brachial index.
1)Values are mean ± SD.
2)Paired t-test
ACKNOWLEDGMENTS
Our sincere appreciation goes to Dongwon F & B Co., Ltd. for providing the folic acid tablets, and all the subjects who participated in this study.
Notes
References
1. Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998; 21:1167–1172.

2. de Oliveira Alvim R, Santos PC, Musso MM, de Sá Cunha R, Krieger JE, Mill JG, Pereira AC. Impact of diabetes mellitus on arterial stiffness in a representative sample of an urban Brazilian population. Diabetol Metab Syndr. 2013; 5:45.

3. van de Ree MA, Huisman MV, de Man FH, van der Vijver JC, Meinders AE, Blauw GJ. Impaired endothelium-dependent vasodilation in type 2 diabetes mellitus and the lack of effect of simvastatin. Cardiovasc Res. 2001; 52:299–305.

4. Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol. 2008; 51:264–270.
5. Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with Type 2 diabetes: a large prospective cohort study. Diabet Med. 2008; 25:1295–1301.

6. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998; 316:823–828.

7. Audelin MC, Genest J Jr. Homocysteine and cardiovascular disease in diabetes mellitus. Atherosclerosis. 2001; 159:497–511.

8. Pfanzagl B, Tribl F, Koller E, Möslinger T. Homocysteine strongly enhances metal-catalyzed LDL oxidation in the presence of cystine and cysteine. Atherosclerosis. 2003; 168:39–48.

9. Jin L, Caldwell RB, Li-Masters T, Caldwell RW. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J Physiol Pharmacol. 2007; 58:191–206.
10. Patterson S, Scullion SM, McCluskey JT, Flatt PR, McClenaghan NH. Prolonged exposure to homocysteine results in diminished but reversible pancreatic β-cell responsiveness to insulinotropic agents. Diabetes Metab Res Rev. 2007; 23:324–334.

11. Patterson S, Flatt PR, McClenaghan NH. Major metabolic homocysteine-derivative, homocysteine thiolactone, exerts changes in pancreatic beta-cell glucose-sensing, cellular signal transduction and integrity. Arch Biochem Biophys. 2007; 461:287–293.

12. Wouters MG, Moorrees MT, van der Mooren MJ, Blom HJ, Boers GH, Schellekens LA, Thomas CM, Eskes TK. Plasma homocysteine and menopausal status. Eur J Clin Invest. 1995; 25:801–805.

13. El-Kadi MA, Farag AF. The effect of folic acid supplementation on serum homocysteine of egyptian post menopausal women: a randomized controlled trial. Middle East Fertil Soc J. 2014; 19:192–196.

14. Qin X, Xu M, Zhang Y, Li J, Xu X, Wang X, Xu X, Huo Y. Effect of folic acid supplementation on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012; 222:307–313.

15. Chambers JC, Ueland PM, Obeid OA, Wrigley J, Refsum H, Kooner JS. Improved vascular endothelial function after oral B vitamins: an effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000; 102:2479–2483.

16. Paradisi G, Cucinelli F, Mele MC, Barini A, Lanzone A, Caruso A. Endothelial function in post-menopausal women: effect of folic acid supplementation. Hum Reprod. 2004; 19:1031–1035.

17. Villa P, Perri C, Suriano R, Cucinelli F, Panunzi S, Ranieri M, Mele C, Lanzone A. L-folic acid supplementation in healthy postmenopausal women: effect on homocysteine and glycolipid metabolism. J Clin Endocrinol Metab. 2005; 90:4622–4629.

18. Sultan N, Khan MA, Malik S. Effect of folic acid supplementation on homocysteine level in postmenopausal women. J Ayub Med Coll Abbottabad. 2007; 19:78–81.
19. Mangoni AA, Sherwood RA, Asonganyi B, Swift CG, Thomas S, Jackson SH. Short-term oral folic acid supplementation enhances endothelial function in patients with type 2 diabetes. Am J Hypertens. 2005; 18:220–226.

20. Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008; 299:2027–2036.

21. Kim H, Park S, Yang H, Choi YJ, Huh KB, Chang N. Association between fish and shellfish, and omega-3 PUFAs intake and CVD risk factors in middle-aged female patients with type 2 diabetes. Nutr Res Pract. 2015; 9:496–502.

22. Organisation for Economic Co-operation and Development. Cardiovascular Disease and Diabetes: Policies for Better Health and Quality of Care. Paris: Organisation for Economic Co-operation and Development;2015. p. 111–131.
23. Durga J, Bots ML, Schouten EG, Grobbee DE, Kok FJ, Verhoef P. Effect of 3 y of folic acid supplementation on the progression of carotid intima-media thickness and carotid arterial stiffness in older adults. Am J Clin Nutr. 2011; 93:941–949.

24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.

25. Lauer M, Clarke R. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics. 1988; 82:309–318.
26. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 25:359–364.

27. Kweon SS, Shin MH, Park KS, Nam HS, Jeong SK, Ryu SY, Chung EK, Choi JS. Distribution of the ankle-brachial index and associated cardiovascular risk factors in a population of middle-aged and elderly koreans. J Korean Med Sci. 2005; 20:373–378.

28. Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF, Masana L, Mangas A, Hernández-Mijares A, González-Santos P, Ascaso JF, Pedro-Botet J. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009; 5:757–765.
29. da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo). 2008; 63:427–432.
30. Keser I, Ilich JZ, Vrkić N, Giljević Z, Colić Barić I. Folic acid and vitamin B(12) supplementation lowers plasma homocysteine but has no effect on serum bone turnover markers in elderly women: a randomized, double-blind, placebo-controlled trial. Nutr Res. 2013; 33:211–219.

31. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. American Heart Association Council on Peripheral Vascular Disease. Council on Epidemiology and Prevention. Council on Clinical Cardiology. Council on Cardiovascular Nursing. Council on Cardiovascular Radiology and Intervention. Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012; 126:2890–2909.

32. Wu L, Wang Y, Zheng L, Li J, Hu D, Xu Y, Hasimu B, Yuan H, Yang J, Sun Y, Ma Y. Distribution of brachial-ankle pulse wave velocity values and optimal cut-off in distinguishing subjects with clinical condition in Chinese population. Int Angiol. 2012; 31:252–259.
33. Tehlivets O. Homocysteine as a risk factor for atherosclerosis: is its conversion to s-adenosyl-L-homocysteine the key to deregulated lipid metabolism? J Lipids. 2011; 2011:702853.
34. Young IS, Woodside JV. Folate and homocysteine. Curr Opin Clin Nutr Metab Care. 2000; 3:427–432.

36. Bostom AG, Shemin D, Lapane KL, Nadeau MR, Sutherland P, Chan J, Rozen R, Yoburn D, Jacques PF, Selhub J, Rosenberg IH. Folate status is the major determinant of fasting total plasma homocysteine levels in maintenance dialysis patients. Atherosclerosis. 1996; 123:193–202.

37. Title LM, Ur E, Giddens K, Mcqueen MJ, Nassar BA. Folic acid improves endothelial dysfunction in type 2 diabetes--an effect independent of homocysteine-lowering. Vasc Med. 2006; 11:101–109.

38. Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, Yang F, Durante W, Chan L, Schafer AI, Pownall HJ, Yang X, Wang H. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006; 99:598–606.

39. Namekata K, Enokido Y, Ishii I, Nagai Y, Harada T, Kimura H. Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. J Biol Chem. 2004; 279:52961–52969.

40. Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001; 107:1263–1273.

41. Adaikalakoteswari A, Jayashri R, Sukumar N, Venkataraman H, Pradeepa R, Gokulakrishnan K, Anjana RM, McTernan PG, Tripathi G, Patel V, Kumar S, Mohan V, Saravanan P. Vitamin B12 deficiency is associated with adverse lipid profile in Europeans and Indians with type 2 diabetes. Cardiovasc Diabetol. 2014; 13:129.

42. Shargorodsky M, Boaz M, Pasternak S, Hanah R, Matas Z, Fux A, Beigel Y, Mashavi M. Serum homocysteine, folate, vitamin B12 levels and arterial stiffness in diabetic patients: which of them is really important in atherogenesis? Diabetes Metab Res Rev. 2009; 25:70–75.

43. Nappo F, De Rosa N, Marfella R, De Lucia D, Ingrosso D, Perna AF, Farzati B, Giugliano D. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999; 281:2113–2118.

44. el-Swefy SE, Ali SI, Asker ME, Mohamed HE. Hyperhomocysteinaemia and cardiovascular risk in female ovariectomized rats: role of folic acid and hormone replacement therapy. J Pharm Pharmacol. 2002; 54:391–397.

45. Xia XS, Li X, Wang L, Wang JZ, Ma JP, Wu CJ. Supplementation of folic acid and vitamin B12 reduces plasma levels of asymmetric dimethylarginine in patients with acute ischemic stroke. J Clin Neurosci. 2014; 21:1586–1590.

46. Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010; 6:82–90.

47. Protopsaltis I, Foussas S, Angelidi A, Gritzapis A, Sergentanis T, Matsagos S, Tzirogiannis K, Panoutsopoulos GI, Dimitriadis G, Raptis S, Melidonis A. Impact of ADMA, endothelial progenitor cells and traditional cardiovascular risk factors on pulse wave velocity among prediabetic individuals. Cardiovasc Diabetol. 2012; 11:141.

48. Koyama K, Ito A, Yamamoto J, Nishio T, Kajikuri J, Dohi Y, Ohte N, Sano A, Nakamura H, Kumagai H, Itoh T. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am J Kidney Dis. 2010; 55:1069–1078.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download