This article has been corrected. See "Erratum: Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice" in Volume 11 on page 435.
Abstract
BACKGROUND/OBJECTIVES
Changes in nutritional status during gestation and lactation have detrimental effects on offspring metabolism. Several animal studies have shown that maternal high-fat diet (HFD) can predispose the offspring to development of obesity and metabolic diseases, however the mechanisms underlying these transgenerational effects are poorly understood. Therefore, we examined the effect of maternal HFD consumption on metabolic phenotype and hepatic expression of involved genes in dams to determine whether any of these parameters were associated with the metabolic outcomes in the offspring.
MATERIALS/METHODS
Female C57BL/6 mice were fed a low-fat diet (LFD: 10% calories from fat) or a high-fat diet (HFD: 45% calories from fat) for three weeks before mating, and during pregnancy and lactation. Dams and their male offspring were studied at weaning.
RESULTS
Dams fed an HFD had significantly higher body and adipose tissue weights and higher serum triglyceride and cholesterol levels than dams fed an LFD. Hepatic lipid levels and mRNA levels of genes involved in lipid metabolism, including LXRα, SREBP-2, FXR, LDLR, and ABCG8 were significantly changed by maternal HFD intake. Significantly lower total liver DNA and protein contents were observed in dams fed an HFD, implicating the disturbed liver adaptation in the pregnancy-related metabolic demand. HFD feeding also induced significant oxidative stress in serum and liver of dams. Offspring of dams fed an HFD had significantly higher serum cholesterol levels, which were negatively correlated with liver weights of dams and positively correlated with hepatic lipid peroxide levels in dams.
A growing body of evidence suggests that the intrauterine environment is an important determinant of fetal growth and development in both animals and humans [123]. In particular, maternal dietary composition and intake have lasting effects on the long-term health of offspring by altering growth and functional development of various organs [3]. In addition, pervasive effects of maternal diet on hepatic gene expression of offspring may program the homeostatic mechanisms in offspring [4].
Several studies have demonstrated a strong relationship between maternal overnutrition and metabolic disorders, including abnormal glucose and lipid metabolism, and adiposity in the offspring, however the mechanisms controlling these transgenerational effects are not fully understood [56]. In addition, previous studies have reported inconsistent results regarding the effects of maternal high-fat diet (HFD) on physiology of dams and their offspring. Several studies reported similar [78], or even lower body weight [9] of offspring of dams fed an HFD compared to those of dams fed a low-fat diet (LFD), which may be due to divergent dietary compositions, feeding duration, species and strain of animals, and age and sex of offspring.
To fulfill the metabolic demands of the developing placenta and fetus, several adaptive responses are induced in maternal organ systems and functions during pregnancy [1011]. In particular, significant maternal liver growth occurs through coordinated increases in the number and size of hepatocytes [1012]. As a major regulatory organ of cholesterol metabolism, hepatic biosynthesis, uptake and efflux of cholesterol must be coordinated to maintain homeostasis for both dams and offspring during pregnancy and lactation period [1314]. Therefore, high-fat feeding, which changes maternal food intake, body composition, hormone and adipokine levels, glucose and lipid metabolism, and placental nutrient transport [3], may result in further increases in metabolic burden of the maternal liver. However, hepatic adjustments to pregnancy in dams fed a metabolically challenging diet are poorly understood [12].
Therefore, in the current study, we examined the effect of maternal HFD consumption for three weeks before mating, and during pregnancy and lactation on metabolic phenotype and hepatic expression of involved genes in dams to determine whether any of these parameters were associated with the metabolic outcomes in male mice offspring.
Four-week-old virgin female C57BL/6 mice were obtained from the local animal facility (Orient Bio Co., Seongnam, Gyunggi-do, Korea) and were maintained in a temperature (23 ± 2℃) and humidity (55 ± 5%)-controlled room with a 12 h dark/light cycle. The experimental procedures used in the current study were approved by Seoul National University Institutional Animal Care and Use Committee (SNU-140807-1-3). After a one-week acclimation period, mice were randomly assigned to one of two groups. The LFD group was fed an LFD (10% calories from fat, 20% from protein, and 70% from carbohydrate, 3.85 kcal/g; #D12450B, Research Diets, Inc., New Brunswick, NJ, USA) and the HFD group was fed an HFD (45% calories from fat, 20% from protein, and 35% from carbohydrate, 4.73 kcal/g; #D12451, Research Diets, Inc., New Brunswick, NJ, USA). Experimental diets were provided for 3 weeks before mating and throughout pregnancy and lactation. Diets and water were provided ad libitum. Females were allowed to mate with mature males (2:1) and the litter size and pup body weights were recorded on postnatal day 3 (PD 3). Out of the 20 females in each group, 14 females in the LFD group and 9 females in the HFD group became pregnant. Lower pregnancy rate in dams fed an HFD was previously reported [1516]. Litters were adjusted to approximately 6 to normalize the growth. Dams (LFD and HFD groups; n = 7 and 5, respectively) and their male offspring at PD 21 (LFD-O and HFD-O groups; n = 10 and 7, respectively) were sacrificed after an overnight fast. Some dams were excluded due to the different sacrifice time point (postpartum day 2), lack of male offspring, or cannibalization of offspring. Some offspring used in the separate long-term intervention study were not sacrificed. Blood samples were obtained rapidly by cardiac puncture. Tissues, including liver, brain, and adipose tissues in dams and liver, brain, spleen, kidney, and adipose tissues in offspring were removed, washed with phosphate-buffered saline (PBS), and weighed. Livers were snap-frozen immediately in liquid nitrogen and stored at -80℃ for further analysis.
Blood was centrifuged at 3,000 rpm for 20 min at 4℃ and stored at -80℃ until analyzed. Serum glucose, triglyceride, total cholesterol and HDL-cholesterol levels were determined using commercial kits (Asan Pharmaceutical Co., Seoul, Korea). Alanine aminotransferase (ALT) activity was measured using the commercial kit (Asan Pharmaceutical Co., Seoul, Korea) and monocyte chemoattractant protein 1 (MCP-1) levels were analyzed using the Quantikine® mouse MCP-1 Immunoassy kit (R&D Systems, Minneapolis, MN, USA). Serum thiobarbituric acid reactive substances (TBARS) levels were measured according to the method of Ohkawa et al. [17]. The absorbance of the butanol layer was measured at 532 nm using 1,1,3,3-tetraethoxypropane as a standard. Serum total protein levels were measured using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA).
Total lipids were extracted according to the method of Folch et al. [18]. Hepatic triglyceride and cholesterol concentrations were determined by enzymatic colorimetric methods using commercial kits (Asan Pharmaceutical Co., Seoul, Korea). Hepatic TBARS was measured using the same method for serum TBARS. The hepatic lipid peroxide level was expressed as malondialdehyde equivalents per milligram of protein and the protein content of the homogenate was measured using a protein assay kit (Bio-Rad, Hercules, CA, USA). DNA was extracted using a DNeasy blood & tissue kit (Qiagen, Valencia, CA, USA).
Liver tissues were homogenized in 10 volumes (w:v) of ice-cold protein lysis buffer using the Tissue Lyser system (Qiagen, Valencia, CA, USA) with 5 mm sterile stainless steel beads. Thirty micrograms of protein were loaded into the lanes of a SDS-PAGE gel, separated, and blotted onto a PVDF membrane. After blocking with 5% nonfat milk or bovine serum albumin in TTBS, membranes were probed with a specific primary antibody, including proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), cleaved form of caspase-3 (Cell signaling Technology, Danvers, MA, USA) or anti-70-kDa heat shock cognate protein (HSC70; Cell signaling Technology, Danvers, MA, USA). The membranes were then incubated with an IgG-peroxidase-conjugated secondary antibody for chemiluminescent detection. The band intensities were quantified using Quantity One software (Bio-Rad, Hercules, CA, USA).
Total RNA of liver tissue was isolated using RNAiso Plus (Takara Bio Inc., Otsu, Shiga, Japan). cDNA was synthesized using 2 mg of total RNA with the Superscript®II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) for qRT-PCR. All amplification reactions were performed using a StepOne™ Real Time PCR System (Applied Biosystems, Foster city, CA, USA) according to the manufacturer's protocol. Mouse ribosomal protein L19 (RPL19) was used as a reference gene and relative gene expression levels were analyzed using the 2-ΔΔCt method. Primers for heme oxygenase 1 (HO-1; F 5'-CCTCACTGGCAGGAA ATCATC-3', R 5'-CCTCGTGGAGACGCTTTACATA-3'), interleukin-1 β (IL-1β; F 5'-CAACCAACAAGTGATATTCTCCATG-3', R 5'-ATCCAC ACTCTCCAGCTGCA-3'), p40phox (F 5'-CCTGCCCACATTGCCAGC CA-3', R 5'-AGACCGGCAGGCTCAGGAGG-3'), and scavenger receptor B1 (SR-B1; F 5'-CCTTCAATGACAACGACACCG-3', R 5'-CCATGCGACTTGTCAGGCT-3') were used for amplification of DNA. Primer sequences for ATP-binding cassette sub-family A member 1 (ABCA1), ABCG5, ABCG8, cluster of differentiation 36 (CD36), carnitine palmitoyltransferase 1A (CPT1a), cholesterol 7 alpha-hydroxylase (CYP7A1), fatty acid synthase (FASN), farnesoid X receptor (FXR), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR), low density lipoprotein receptor (LDLR), liver X receptor α (LXRα), MCP-1, stearoyl-CoA desaturase-1 (SCD1), SR-A, sterol regulatory element-binding protein 2 (SREBP-2), peroxisome proliferator-activated receptor α (PPARα), PPARγ, tumor necrosis factor α (TNFα), and RPL19 were previously published [19].
Statistical analyses were performed using SPSS version 22 software (IBM SPSS Inc., Chicago, Il, USA). Student t-test was used to determine statistical significance between two groups for all experiments. Data were expressed as mean ± SEM and differences were considered statistically significant at P < 0.05. Correlations between two variables were determined by Pearson's correlation coefficient.
There were no significant differences in maternal body weights measured at baseline, mating, and postpartum day 3 between groups (Fig. 1). In terms of total daily calorie intake, dams under an HFD consumed more calories than dams fed an LFD before mating, and during pregnancy and lactation (data not shown). Maternal consumption of HFD increased body weight and adipose tissue weight of dams at the end of the study (21 days after giving birth to offspring) (Table 1). Interestingly, a higher liver weight was observed in the LFD group compared to the HFD group. Significantly higher serum lipid parameters including triglyceride and total cholesterol were observed in HFD-fed dams (Fig. 2A). No significant difference in glucose, HDL-cholesterol, total protein, and ALT levels in the sera was observed between groups. HFD feeding significantly led to increased hepatic triglyceride and decreased cholesterol levels in dams (Fig. 2B).
Because altered lipid levels were observed in both serum and liver of dams fed an HFD, mRNA levels of hepatic genes involved in lipid metabolism were determined. There were no significant differences in major transcription factors and most enzymes involved in triglyceride metabolism (Fig. 3A). Interestingly, significantly lower mRNA levels of SCD1 were observed in the HFD group compared with the LFD group, suggesting the increased accumulation and VLDL incorporation of saturated fatty acids in the liver of dams fed an HFD as previously reported [2021]. There were significant differences in mRNA levels of major transcription factors involved in cholesterol metabolism, including LXRα, SREBP-2, and FXR, and their target genes including ABCG8 and LDLR (Fig. 3B & C).
Due to the increased metabolic demand during pregnancy, the maternal liver grows by hyperplasia and hypertrophy [10]. Therefore, total liver DNA and protein contents were determined to examine whether dysregulated hepatic proliferation and growth during pregnancy may result in lower liver weights in the HFD group. As shown in Fig. 4A, higher DNA and protein contents were observed in dams fed an LFD. The catch-up in liver growth during the lactation period was not observed in dams fed an HFD based on PCNA and active form of capase-3 protein levels (Fig. 4B).
Hypercholesterolemia has been suggested as an important risk factor for hepatic inflammation in animal models [19]. Therefore, we examined the effects of HFD feeding on oxidative stress and inflammation in dams. Significantly higher hepatic serum and lipid peroxide levels were observed in dams fed an HFD (Fig. 5A). Significantly higher hepatic mRNA levels of HO-1 and p40phox, enzymes involved in cellular antioxidant capacity, were observed in dams fed an LFD (Fig. 5B).
Although serum MCP-1 levels were not significantly different between groups (Fig. 5C), significantly higher expression of hepatic MCP-1 was observed in dams fed an HFD compared to dams fed an LFD. Expression of other inflammatory genes, including TNFα and CD68, was not significantly different between groups (data not shown).
Maternal HFD feeding did not change litter size, and the body weights of offspring were not significantly different between the two groups until weaning (data not shown). Maternal diet had no significant effects on offspring organ weights including liver, adipose tissue, brain, spleen, and kidney (Table 2).
Significantly higher serum total cholesterol levels, but not serum glucose, triglyceride, and ALT levels, were observed in the offspring from HFD-fed dams (HFD-O group) compared to the offspring from LFD-fed dams (LFD-O group) (Fig. 6A). Interestingly, serum total cholesterol levels of offspring were negatively correlated with liver weights of dams and positively correlated with hepatic TBARS levels of dams (Fig. 6B). No significant difference in hepatic triglyceride and cholesterol levels was observed between groups (data not shown).
Previous studies have reported that maternal HFD consumption interferes with liver development in offspring, which may induce development of liver disease later in life [82223]. Although no difference in relative liver weight was observed, additional biomarkers of liver development were determined in order to confirm the effect of maternal HFD on liver development in offspring. As shown in Fig. 7, no significant differences in cell proliferation, growth, and apoptosis in the liver were observed between the two groups.
Because significantly higher serum cholesterol levels were observed in the HFD-O group compared with the LFD-O group, the hepatic expression of genes involved in cholesterol metabolism was determined. Expression levels of most genes were not significantly different, however significant differences in mRNA levels of ABCG8, which promotes excretion of cholesterol into bile, were observed between the two groups (Fig. 8). No significant differences were observed in hepatic expression of genes involved in oxidative stress and inflammation (data not shown).
In the current study, female C57BL/6 mice were fed either an LFD or an HFD for three weeks before mating throughout pregnancy and lactation to examine the effect of maternal HFD consumption on metabolic phenotype and hepatic expression of involved genes in dams to determine whether any of these parameters were associated with the metabolic outcomes in the offspring. Altered triglyceride and cholesterol metabolism was observed in dams fed an HFD and altered cholesterol metabolism was observed in their offspring. In addition, maternal HFD intake disturbed liver growth and increased oxidative stress in dams, which may contribute to the deregulation of cholesterol homeostasis of their offspring.
Due to the increasing metabolic demands, maternal hepatocytes undergo proliferation and size reduction and the size of hepatocytes then increases without replication during pregnancy [10]. Sustained growth of liver during lactation has been demonstrated [12]. Interestingly, a lower liver weight was observed in dams fed an HFD possibly due to the impaired liver growth based on total liver DNA and protein contents. A significant catch-up in liver growth of dams on postpartum day 21 was not observed based on PCNA immunoblotting. Therefore, disrupted liver growth during pregnancy may result in alterations in hepatic metabolism of lipid and reactive oxygen species as observed in the current study.
Previous studies have reported that perinatal oxidative stress plays an important role in programming the susceptibility of offspring to metabolic diseases and that adequate maternal antioxidant status before and during pregnancy regulates pregnancy outcomes [2425]. In particular, detrimental effects of oxysterols on the development of the fetus have been suggested [26], and increased pro-inflammatory adipokines in obese dams have been shown to induce oxidative stress and inflammation in the placenta during pregnancy [27]. Maternal consumption of HFD has also been shown to impair the synthesis and secretion of milk from mammary gland, possibly through increased serotonin biosynthesis within the mammary gland leading to an inflammatory process [28].
Our results showed no significant differences in relative liver weight, liver proliferation, growth, and development of offspring at PD 21. A previous study reported inhibition of the Wnt/beta-catenin pathway in male offspring at PD 14 of dams fed a Western-style diet [23]. Because activation of beta-catenin activity during embryonic day 10 to 14 and PD 5 to 20 has been demonstrated [29], the time differences between the two studies might explain the catch-up growth of impaired liver by PD 21 as observed in the current study.
Higher serum cholesterol levels were observed in offspring of dams fed an HFD as reported in previous studies using offspring at PD 21 [930]. Unlike dams, hepatic mRNA levels of ABCG8, but not LXRα, SREBP2, and LDLR, were significantly different between the LFD-O and the HFD-O groups. In a previous study, reporting increased serum total cholesterol levels in offspring, no cholesterol-metabolizing enzyme genes were identified as differentially expressed genes [9]. In another study, reporting lower serum cholesterol levels in PD 14 male offspring of dams fed an HFD, genes related to cholesterol transport, but not other cholesterol metabolism, were significantly up-regulated in PD 14 male offspring of dams fed an HFD [23] implicating the pivotal role of hepatic cholesterol transporter protein, including ABCG8, in the regulation of cholesterol homeostasis.
In conclusion, maternal consumption of an HFD disturbed liver growth and increased oxidative stress in dams, which appeared to be involved in the altered cholesterol metabolism in offspring at PD 21. Therefore, dietary and supplementary intake of antioxidants before and during pregnancy may have beneficial effects on cholesterol metabolism in offspring of dams fed an HFD.
Figures and Tables
 | Fig. 1Effects of maternal HFD consumption on body weight of dams.Body weights were measured at baseline, mating, postpartum day (PP) 3 and PP20. Data are presented as mean ± SEM (LFD: n = 7, HFD: n = 5). *Significantly different from the LFD group (P < 0.05 by Student's t-test).
|
 | Fig. 2Effects of maternal HFD consumption on serum and hepatic biochemical parameters of dams.(A) Serum glucose, triglyceride, total cholesterol, HDL-cholesterol, ALT, and protein levels. (B) Hepatic triglyceride and cholesterol levels. Data are presented as mean ± SEM (LFD: n = 7, HFD: n = 5). *Significantly different from the LFD group (P < 0.05 by Student's t-test).
|
 | Fig. 3Effects of maternal HFD consumption on hepatic mRNA levels of genes involved in (A) triglyceride metabolism, (B) cholesterol metabolism, and (C) lipoprotein transport in dams.Relative mRNA expression was analyzed by real-time PCR and normalized to RPL19 as endogenous control. Data are presented as mean ± SEM (LFD: n = 7, HFD: n = 5). *Significantly different from the LFD group (P < 0.05 by Student's t-test).
|
 | Fig. 4Effects of maternal HFD consumption on liver growth and proliferation of dams.(A) Total liver DNA and protein contents. Data are presented as mean ± SEM (LFD: n = 7, HFD: n = 5). *Significantly different from the LFD group (P < 0.05 by Student's t-test). (B) Representative immunoblots of hepatic PCNA and cleaved caspase-3. Each protein was normalized to HSC70.
|
 | Fig. 5Effects of maternal HFD consumption on oxidative stress and inflammation of dams.(A) Serum and hepatic TBARS levels. (B) Hepatic mRNA levels of p40phox and HO-1. (C) Serum MCP-1 levels and hepatic mRNA levels of MCP-1. Relative mRNA expression was analyzed by real-time PCR and normalized to RPL19 as endogenous control. Data are presented as mean ± SEM (LFD: n = 7, HFD: n = 5). * Significantly different from the LFD group (P < 0.05 by Student's t-test).
|
 | Fig. 6Effects of maternal HFD consumption on serum biochemical parameters of male offspring.(A) Serum glucose, triglyceride, total cholesterol and ALT levels. Data are presented as mean ± SEM (LFD-O: n = 10, HFD-O: n = 7). * Significantly different from the LFD-O group (P < 0.05 by Student's t-test). (B) Correlation between offspring serum cholesterol levels and the relative liver weight of dams. (C) Correlation between offspring serum cholesterol levels and hepatic TBARS levels of dams.
|
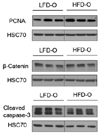 | Fig. 7Effects of maternal HFD consumption on hepatic PCNA, β-catenin, and cleaved caspase-3 protein levels of male offspring.Representative immunoblot of each protein was normalized to HSC70.
|
 | Fig. 8Effects of maternal HFD consumption on hepatic mRNA levels of genes involved in cholesterol metabolism of male offspring.Relative mRNA expression was analyzed by real-time PCR and normalized to RPL19 as endogenous control. Data are presented as mean ± SEM (LFD-O: n = 10, HFD-O: n = 7). * Significantly different from the LFD-O group (P < 0.05 by Student's t-test).
|
References
1. Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010; 30:315–339.

3. Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta. 2014; 1842:507–519.

4. Cannon MV, Buchner DA, Hester J, Miller H, Sehayek E, Nadeau JH, Serre D. Maternal nutrition induces pervasive gene expression changes but no detectable DNA methylation differences in the liver of adult offspring. PLoS One. 2014; 9:e90335.

5. Cianfarani S, Agostoni C, Bedogni G, Berni Canani R, Brambilla P, Nobili V, Pietrobelli A. Effect of intrauterine growth retardation on liver and long-term metabolic risk. Int J Obes (Lond). 2012; 36:1270–1277.

6. Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010; 299:R711–R722.

7. Bringhenti I, Ornellas F, Martins MA, Mandarim-de-Lacerda CA, Aguila MB. Early hepatic insult in the offspring of obese maternal mice. Nutr Res. 2015; 35:136–145.

8. Yang KF, Cai W, Xu JL, Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol. 2012; 49:107–114.

9. Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the PPAR pathway in the early life of offspring. Int J Mol Sci. 2014; 15:14967–14983.

10. Zou Y, Hu M, Bao Q, Chan JY, Dai G. Nrf2 participates in regulating maternal hepatic adaptations to pregnancy. J Cell Sci. 2013; 126:1618–1625.

11. Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000; 54:Suppl 1. S47–S51.

12. Bustamante JJ, Copple BL, Soares MJ, Dai G. Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int. 2010; 30:406–415.

13. Papacleovoulou G, Abu-Hayyeh S, Williamson C. Nuclear receptor-driven alterations in bile acid and lipid metabolic pathways during gestation. Biochim Biophys Acta. 2011; 1812:879–887.

14. Athippozhy A, Huang L, Wooton-Kee CR, Zhao T, Jungsuwadee P, Stromberg AJ, Vore M. Differential gene expression in liver and small intestine from lactating rats compared to age-matched virgin controls detects increased mRNA of cholesterol biosynthetic genes. BMC Genomics. 2011; 12:95.

15. Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009; 27:627–633.

16. Shaw MA, Rasmussen KM, Myers TR. Consumption of a high fat diet impairs reproductive performance in Sprague-Dawley rats. J Nutr. 1997; 127:64–69.

17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.

18. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.

19. Jeon S, Park YJ, Kwon YH. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(-/-) mice fed a high-fat diet. Mol Nutr Food Res. 2014; 58:830–841.

20. Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, Keller MP, Ntambi JM, Attie AD. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007; 56:1228–1239.

21. Matsui H, Yokoyama T, Sekiguchi K, Iijima D, Sunaga H, Maniwa M, Ueno M, Iso T, Arai M, Kurabayashi M. Stearoyl-CoA desaturase-1 (SCD1) augments saturated fatty acid-induced lipid accumulation and inhibits apoptosis in cardiac myocytes. PLoS One. 2012; 7:e33283.

22. Dudley KJ, Sloboda DM, Connor KL, Beltrand J, Vickers MH. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011; 6:e21662.

23. Mischke M, Pruis MG, Boekschoten MV, Groen AK, Fitri AR, van de Heijning BJ, Verkade HJ, Müller M, Plösch T, Steegenga WT. Maternal Western-style high fat diet induces sex-specific physiological and molecular changes in two-week-old mouse offspring. PLoS One. 2013; 8:e78623.

24. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010; 42:1634–1650.

25. Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010; 59:3058–3065.

26. Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plösch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod. 2013; 88:24.
27. Ashino NG, Saito KN, Souza FD, Nakutz FS, Roman EA, Velloso LA, Torsoni AS, Torsoni MA. Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J Nutr Biochem. 2012; 23:341–348.

28. Hernandez LL, Grayson BE, Yadav E, Seeley RJ, Horseman ND. High fat diet alters lactation outcomes: possible involvement of inflammatory and serotonergic pathways. PLoS One. 2012; 7:e32598.

29. Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G1578–G1585.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download