Abstract
Solar ultraviolet (UV) irradiation leads to distinct changes in the skin connective tissues by degradation of collagen, which is a major structural component in the extracellular matrix. UV irradiation induces the production of matrix metalloproteinases (MMP) capable of attacking native fibrillar collagen and responsible for inhibiting the construction of collagenous extracellular matrix. In this study, we attempted to investigate the protective actions of Rubus coreanus ethanol extract (RCE) on the MMP production and the consequent procollagen/collagen degradation in UV-B-irradiated human dermal fibroblasts. The analytical data showed that Rubus coreanus ethanol extract was mostly comprised of cyanidin 3-rutinoside. Pre-treatment of fibroblasts with this extract inhibited UV-B-induced production of MMP-1, MMP-8 and MMP-13 in dose-dependent manners. In addition, Western blot analysis and immunocytochemical staining assay revealed that RCE markedly augmented the cellular levels of procollagen/collagen declined in UV-B-exposed dermal fibroblasts. These results demonstrate that RCE blocks UV-B-induced increase of the collagen degradation by inhibiting MMP production. Thus, RCE may act as an agent inhibiting excessive dermal collagen degradation leading to the skin photoaging.
Ultraviolet (UV) irradiation leads to distinct changes in the skin connective tissue by degradation of collagen, which is a major component in the extracellular matrix (Offord et al., 2002; Saito et al., 2004). These alterations in the extracellular matrix most likely mediated by matrix metalloproteinases (MMP) appear to cause the skin wrinkling observed in the skin rash. Human dermal tissues express a number of MMP including MMP-1 (interstitial collagenase), MMP-8 (neutrophil collagenase) and MMP-13 (collagenase-3), all of which are capable of attacking native fibrillar collagen (Varani et al., 2002). Since collagen fibrils and elastin predominantly present in the skin are responsible for the strength and resiliency of skin, their disarrangement during photoaging causes the skin to appear aged (Huang et al., 1997). UV irradiation causes direct and indirect DNA damage, formation of reactive oxygen species (ROS) and associated inflammatory responses and damage to the extracellular matrix (Harman et al., 1992). These ROS could result in the subsequent activation of complex signaling pathway, followed by MMP induction in dermal cells (Fisher & Voorhees, 1998; Oh et al., 2004).
Rubus coreanus has been used for centuries as traditional alternative medicines. Dried fruits of this plant are used as a promising agent reducing the risk of many diseases including asthma and allergic diseases, and are effective in dampening inflammation and oxidation (Lee, 1996; Moon, 1991). Moreover, it was shown that Rubus coreanus extract enhanced the osteoblast function by promoting cell differentiation and inhibiting bone-resorbing mediators (Lee & Choi, 2006). Although several studies demonstrated the biological functions of Rubus coreanus (Lee, 1996; Lee & Choi, 2006; Moon, 1991), there is little known for bioactive components isolated from Rubus coreanus. Nigaichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus coreanus have anti-gastropathic and anti-rheumatic effects (Choi et al., 2003; Nam et al., 2006). In addition, it was reported that ellagic acid in Rubus species possesses anti-carcinogenic and anti-leukaemic features (Narayanan et al., 1999; Paivarinta et al., 2006; Skupien et al., 2006).
Based on the literature evidence that UV-induced dermal collapse is an initial event in the development of photoaging, this study attempted to investigate inhibitory effects of Rubus coreanus ethanol extract (RCE) on secretion of collagenolytic MMP-1, MMP-8 and MMP-13 in UV-B-exposed human dermal fibroblasts. It was further assessed whether the UV-B-induced increase in the collagen degradation was attenuated by RCE.
Human primary dermal fibroblasts were obtained from Clonetics (San Diego, CA). Dulbecco's modified eagle's media (DMEM) and human β-actin antibody were obtained from Sigma-Aldrich Chemical (St. Louis, MO), as were all other reagents, unless specifically stated elsewhere. Fetal bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA were purchased from Cambrex Corporation (East Rutherford, NJ). 3-(4,5-Dimethylthiazol-yl)-diphenyl tetrazolium bromide (MTT) was purchased from Duchefa Biochemie (Haarlem, Netherlands). Antibodies against human MMP-1, human MMP-8, human MMP-13, human type1 collagen, and human type1 procollagen were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated goat anti-mouse and donkey anti-goat IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Cyanine3-OSu conjugated donkey anti-goat IgG and FITC conjugated rabbit anti-goat IgG were provided from Rockland Co. (Gilbertville, PA) and from Sigma Chemical, respectively.
Ripe fruits of Rubus coreanus Miquel (Rosaceae) were collected from the Hoeng Seong region (Gangwon-do, Korea) on 10 August 2005. The ethanol extracts of Rubus coreanus fruits were prepared. In brief, 50 g lyophilized ripe fruits were pulverized and extracted three times with 1 L of 95% ethanol, generating 42.9% yields.
Tandems mass spectrometry coupled to high-performance liquid chromatography (HPLC) with photo-diode array detection (LC-UV-ESI/MS/MS) analysis was performed using a Thermo Co. LCQ system with a Phenomenex Luna 5 C18 reverse-phase column (4.6×250mm) (Hong & Wrolstad, 1990; Torre & Barritt, 1977). The mobile phases consisted of 2% acetic acid (A) and acetonitrile (B), and were degassed ultrasonically prior to use. For the gradients, an isocratic elute 5% B applied for 2 min was followed by a linear gradient from 5-90% B for 28 min. The column was thermostated at 28℃ and a flow-rate of 0.8 ml/min was used. The final concentrations of the prepared RCE solutions for the HPLC analysis were 5 mg/mL.
Human dermal fibroblasts were cultured in DMEM containing 10% FBS, 2 mM glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin at 37℃ humidified atmosphere of 5% CO2 in air. Cells were plated at 90-95% confluence in all experiments. The UV-B light source (312 nm) was provided from Bio-Sun lamps (Vilber Lourmat, Marine, France). Dermal fibroblasts at a density of 5.0 × 104 cells/well on a 24-well plate were treated with 1-10 µg/mL RCE and exposed to 100 mJ/cm2 UV-B in phosphate-buffered saline (PBS). Subsequently, cells were incubated overnight in DMEM containing equivalent doses of RCE. Crude RCE was dissolved in dimethyl sulfoxide (DMSO) for culturing with cells (Anderson & Garner, 1998), and the final culture concentration of DMSO was ≤0.5%.
At the end of the time incubation period, the MTT assay was performed to quantitate cellular viability (Choi et al., 2005). Human dermal fibroblasts were incubated in a fresh medium containing 1mg/mL MTT for 3 h at 37℃. After removal of unconverted MTT, the purple formazan product was dissolved in 0.5 mL isopropanol through gentle shaking. Absorbance of formazan dye was colorimetrically measured at λ=570 nm.
Western blot analysis was performed using whole cell extracts prepared from human dermal fibroblasts. Whole fibroblast lysates were prepared in a lysis buffer containing 1% β-mercaproethanol, 1 M β-glycerophosphate, 0.1 M Na3VO4, 0.5 M NaF and protease inhibitor cocktail. Cell lysates containing equal amounts of total proteins and equal volumes of culture supernatants were fractionated by electrophoresis on 6% or 10% SDS-PAGE gels and transferred onto a nitrocellulose membrane. Nonspecific binding was blocked by soaking the membrane in a TBS-T buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween 20] containing 3% bovine serum albumin for 3 h. The membrane was incubated with monoclonal mouse anti-human MMP-1 (1:100), polyclonal goat anti-human MMP-8 (1:100), polyclonal goat anti-human MMP-13 (1:100), polyclonal goat anti-human type 1 collagen (1:100), or type 1 procollagen (1:100). Subsequently, the membrane was then incubated with a goat anti-mouse IgG (1:2,000) or donkey anti-goat IgG (1:10,000) conjugated to horseradish peroxidase. The protein levels were determined by using Supersignal West Pico Chemiluminescence detection reagents (Pierce Biotech. Inc., Rockford, IL) and Konica X-ray film (Konica Co., Tokyo, Japan). Incubation with polyclonal mouse anti-human β-actin antibody (1:1,000) was performed for comparative control.
After human dermal fibroblasts grown on 24-well chamber slides were thoroughly washed with PBS containing 0.05% Tween 20, cells were fixed with 4% ice-cold formaldehyde for 30 min and treated for 2 min with 0.1% Triton-X100 and 0.1% citric acid in PBS. For blocking any nonspecific binding, cells were incubated for 1 h with 20% FBS.
-In situ detection of type 1 procollagen levels: After washing fixed cells with PBS, polyclonal goat anti-human type 1 procollagen antibody (1:100) was sufficiently added to cells and incubated overnight at 4℃. Cells were incubated with FITC conjugated anti-goat IgG (1:10,000) as a secondary antibody.
-In situ detection of type 1 collagen levels: Polyclonal goat anti-human collagen type 1 antibody (1:100) was incubated with fixed cells overnight at 4℃. Cells were incubated with Cyanine 3-OSu conjugated anti-goat IgG (1:10,000) as a secondary antibody.
Fluorescent images were obtained by a fluorescence microscopy with an Olympus BX51 fluorescent microscope with differential interference contrast and reflected light fluorescence.
The results were presented as mean ± SEM for each treatment group of each experiment. Statistical analyses were conducted using Statistical Analysis Systems statistical software package (SAS Institute Inc., Cary, NC). Significance was determined by one-way ANOVA followed by Duncan multiple range test for multiple comparisons and considered significant at p<0.05.
Fig. 1 shows the LC-UV-ESI/MS/MS data of anthocyanins present in crude RCE. UV-Vis analysis recognized the presence of one major anthocyanin (Fig. 1A and 1D). The peak presents a mass for the molecular ion at m/z 595 and fragment ion at m/z 448.9 (Fig. 1B). The MS/MS of m/z 595 produced fragment ions at m/z 448.82, which corresponded to a loss of rhamnose constituent from m/z 595 in addition to the common aglycone cation at m/z 287 (Fig. 1C). The molecular cation, aglycone cation, fragmentation pattern, and UV-Vis spectra of this anthocyanin matched with cyanidin 3-rutinoside (Fig. 1E).
To examine the inhibitory effects of the UV-B irradiation on human dermal fibroblast toxicity, the MTT analysis was performed for the measurement of cell viability. The UV-B irradiation at 100 mJ/cm2 substantially reduced cell viability. When RCE in concentrations between 1 and 10 µg was added to UV-B-exposed dermal fibroblasts, the viability was considerably enhanced; 10 µg RCE was required to achieve a full inhibitory effect in such UV-B dermal damage models (Fig. 2).
Fig. 3 shows the inhibitory effects of RCE on the MMP production in UV-B-induced human dermal fibroblasts. The UV-B irradiation markedly enhanced the production of collagenolytic proteins of MMP-1, MMP-8 and MMP-13. Pre-treatment of UV-B-exposed cells with RCE at doses of 1-10 µg/mL for 48 h attenuated the production of these enzymes in dose-dependent manners. The complete blockade of MMP secretion was observed with ≥5 µg RCE (Fig. 3). In addition, the secretion rates of three different MMP enzymes by UV-B irradiation were in the order of MMP-1 > MMP-8 > MMP-13.
Collagen is synthesized in a precursor form of procollagen and secreted out of the cells. The Western blot data revealed that UV-B exposure caused a marked reduction in the level of procollagen (Fig. 4A). In contrast, the diminished procollagen level was dose-dependently boosted by treating RCE to human dermal fibroblasts. Consistent with the blot data, immunostaining assay using a specific procollagen antibody confirmed that RCE blocked the UV-B-induced reduction at the procollagen level (Fig. 4B). There was a relatively weak staining in UV-B-alone-exposed cells, whereas a heavy staining was observed in RCE-treated and UV-B-exposed cells. The effects were obvious with ≥1 µg/mL RCE, indicating that RCE dose-dependently enhanced the procollagen expression dampened by UV-B.
Western blot analysis and then immunocytochemical assay showed that 100 mJ/cm2 UV-B markedly reduced the collagen levels, indicative of promoted collagen degradation (Fig. 5A and 5B). When 1-10 µg/mL RCE was applied to human dermal fibroblasts exposed to UV-B, it was found that the cellular level of collagen protein was restored within 48 h after the UV-B irradiation. It should be noted that ≥5 µg/mL RCE completely abolished the MMP secretion enhanced by UV-B irradiation (Fig. 3). Accordingly, the augmented MMP production appeared to be responsible for the collagen degradation promoted by UV-B irradiation, which was near-completely reversed by a treatment with ≥5 µg/mL RCE.
Four major observations were extracted from this study. 1) Exposure of human skin fibroblasts to UV-B irradiation up-regulated the secretion of MMP-1, MMP-8 and MMP-13, which was abolished at ≥5 µg/mL RCE. 2) RCE enhanced the procollagen level of human dermal fibroblasts mitigated by UVB irradiation in a dose-dependent manner. 3) The cellular level of collagen was sharply declined in UV-B-exposed fibroblasts, and such drop was restored by treating cells with RCE, indicative of inhibiting dermal collagen breakdown. 4) The crude RCE contained cyanidin-3-rutinoside as a major active component, which appeared to be effective in reducing collagen degradation. These overall observations demonstrate that RCE has the potential capability to prevent UV-B irradiation-induced skin photoaging through blunting collagen degradation and subsequent dermal collapse.
Although the amount of UV-B is far less than UV-A, its capacity to cause sunburn is more than UV-A by 1000 times, and UV-B may cause photoaging and skin cancer by promoting the formation of free radicals and directly damaging DNA (Rogers & Gilchrest, 1990). Collagen protein plays an important role in the skin elasticity, and the skin is aged by various means including chemical and physiological actions and UV irradiation (Chau et al., 2005). It is synthesized in a form of procollagen and secreted out of the cells. While it is secreted, extra propeptides at the edge of the procollagen are separated from the parent by certain protease and converted into collagen (Prockop et al., 1979). This study found that RCE protected collagen from breakdown and enhanced the cellular level of type 1 procollagen dropped by UV-B irradiation.
Among many photochemoprotective agents, botanical constituents appear to be promise and their use may be an effective strategy for reduction of incidence of skin cancer and other UV-mediated oxidative damage (Ho et al., 2005). Numerous studies showed that polyphenols could be potential agents reducing the risk of skin diseases (Offord et al., 2002; Hsu, 2005; Jimenez et al., 2006). Ellagic acid and tannic acid prevented proteolytic degradation of existing dermal elastic fibers and for enhancing more efficient elastogenesis in aged skin (Jimenez et al., 2006). In addition, green tea catechins may be used as chemopreventive, natural healing and anti-aging agents for human skin (Hsu, 2005), and it is assumed that this phenomenon be associated with their antioxidant capacity. Recently, it was shown that the ethanol extract of unripe fruits of Rubus coreanus exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 murine macrophages via induction of heme oxygenase-1 pathways (Park et al., 2006).
The UV-B irradiation-induced ROS production caused skin photoaging concomitant with synthesis of MMP (Fisher & Voorhees, 1998). It was reported that MMP activity was increased in the dermal tissues even when UV was irradiated just one time (Fisher & Voorhees, 1998). Such increase in the MMP activity affected collagen degradation in the dermal layer of the skin and played a crucial role in photoaging. The present study showed that UV-B irradiation at 100 mJ/cm2 resulted in a marked secretion of collagenolytic MMP including MMP-1, MMP-8 and MMP-13 at respective different rates, proving the literature evidence. Subsequently, the collagen degradation was promoted concomitantly with a sharp drop in the cellular levels of procollagen. On the other hand, this study recognized that the single major HPLC peak observed in the crude RCE was cyanidin 3-rutinoside, an anthocyanin. Accordingly, this RCE constituent appeared to be responsible for interrupting collagen degradation and subsequent skin photoaging. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protected human HaCaT keratinocytes and mouse skin against UV-B-mediated oxidative stress and apoptosis (Afaq et al., 2007). In addition, black raspberries, strawberries, and blueberries differed in their ability to influence signaling pathways leading to activation of NF-κB and AP-1 when using UV light as the inducer (Huang et al., 2007). Cyanidin-3-rutinoside found in abundance in black raspberries and not in strawberries or high-bush blueberries, was found to contribute to the inhibition of UV-B-induced activation of NF-κB.
In summary, the UV-B irradiation promoted dermal collagen degradation through increasing collagenolytic MMP production and mitigating the cellular procollagen levels. When microgram doses of RCE were applied to UV-B-exposed fibroblasts, the collagen degradation was almost abolished at ≥5 µg/mL. This inhibitory action of RCE was accomplished mainly by its constituent of cyanidin-3-rutinoside, manifested by the LC-UV-ESI/MS/MS analysis. Therefore, crude RCE may be a beneficial agent for blunting dermal collapse, contributing to the interruption of UV sunlight-associated skin photoaging.
Figures and Tables
Fig. 1
LC-UV-ESI/MS/MS data recognizing cyanidin-3-rutinoside abundant in ripe fruits of Rubus coreanus.
Total ion chromatography (A), mass spectra at 9.10min in TIC (B), tandem mass spectra of [M+H]+ (595) (C), HPLC chromatogram (PDA, photodiode-array detector) (D), ultra-violet spectra at 8.74 min (E) and structure of cyanidin 3-rutinoside (F).

Fig. 2
Cell viability in RCE-treated human dermal fibroblasts challenged with UV-B irradiation.
Confluent fibroblasts were left untreated or stimulated with 100 mJ/cm2 prior to incubation for 48 h with RCE. Cell viability was measured using MTT assay and presented as means ± SEM from 3 independent experiments with multiple estimations. Values not sharing a letter are different at P<0.05.
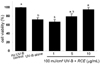
Fig. 3
Inhibitory dose responses of RCE to induction of MMP-1, MMP-8 and MMP-13 in UV-B irradiated human dermal fibroblasts.
After cells were pre-treated with 1-10 µg/mL RCE and exposed to 100 mJ/cm2 UV-B, conditioned culture media were collected and subjected to 10% SDS-PAGE, followed by Western blot analysis with respective primary antibody of MMP-1, MMP-8 and MMP-13. Bands are representative of 3 independent experiments.
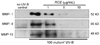
Fig. 4
Inhibitory dose responses of RCE to reduction of type 1 procollagen levels in 100 mJ/cm2 UV-B-irradiated human dermal fibroblasts.
Cell lysates were subjected to 6% SDS-PAGE and Western blot analysis with a primary antibody against type 1 procollagen (A). β-Actin protein was used as an internal control. Bands were representative of 3 independent experiments. In immunocytochemical experiments (B), cells were fixed and then incubated with goat anti-human type 1 procollagen. Antibody localization was detected with FITC conjugated donkey anti-goat IgG using a fluorescence microscopy. Representative fluorescent images were obtained from 3 separate experiments. Magnification: 100-fold.
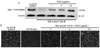
Fig. 5
Augmentation of type 1 collagen levels in RCE-treated and 100 mJ/cm2 UV-B-irradiated human dermal fibroblasts.
Cell lysates were electrophoresed on 6% SDS-PAGE, followed by Western blot analysis with a primary antibody against type 1 collagen (A). β-Actin protein was used as an internal control. Bands were representative of 3 independent experiments. For the immunocytochemical staining (B), fibroblasts were fixed and incubated with goat anti-human type 1 collagen. Antibody localization was detected with Cy-3 conjugated donkey anti-goat IgG using a fluorescence microscopy with rhodamine green filter. Representative fluorescent images were obtained from 3 separate experiments. Magnification: 100-fold.
References
1. Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007. 127:222–232.

2. Anderson JJ, Garner SC. Phytoestrogens and bone. Baillieres Clin Endocrinol Metab. 1998. 12:543–557.
3. Chau DY, Colliqhan RJ, Verderio EA, Addy VL, Griffin M. The cellular response to transglutaminase-cross-linked collagen. Biomaterials. 2005. 26:6518–6529.

4. Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ, Park HJ. Antinociceptive and antiinflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull. 2003. 26:1436–1441.

5. Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005. 135:707–713.

6. Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998. 3:61–68.

8. Ho JN, Lee YH, Park JS, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm Bull. 2005. 28:1244–1248.

9. Hong V, Wrolstad RE. Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J Agric Food Chem. 1990. 38:708–715.

11. Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein-1 activity, but not activation retinoic acid response element, is required for the actitumor promotion effect of retinoic acid. Proc Natl Acad Sci. 1997. 94:5826–5830.

12. Huang C, Zhang D, Li J, Tong Q, Stoner GD. Differential inhibition of UV-induced activation of NF-κB and AP-1 by extracts from black raspberries, strawberries, and blueberries. Nutr Cancer. 2007. 58:205–212.

13. Jimenez F, Mitts TF, Liu K, Wang Y, Hinek A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J Invest Dermatol. 2006. 126:1272–1280.

14. Lee SJ. Korean Folk Medicine. 1996. Seoul, Korea: Publishing Center of Seoul National University.
15. Moon GS. Constituents and Uses of Medicinal Herbs. 1991. Seoul, Korea: Ilweolseogak;310–311.
16. Nam JH, Jung HJ, Choi J, Lee KT, Park HJ. The antigastropathic and anti-rheumatic effect of niga-ichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus coreanus in a rat model. Biol Pharm Bull. 2006. 29:967–970.

17. Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999. 136:215–221.

18. Offord EA, Gautier JC, Avanti O, Scaletta C, Runge F, Kramer K, Applegate LA. Photo-protective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UV A-irradiated human skin fibroblasts. Free Radic Biol Med. 2002. 32:1293–1303.

19. Oh JH, Chung AS, Steinbrenner H, Sies H, Brenneisen P. Thioredoxin secreted upon ultraviolet A irradiation modulates activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Arch Biochem Biophys. 2004. 423:218–226.

20. Paivarinta E, Pajari AM, Torronen R, Mutanen M. Ellagic acid and natural sources of ellagitannins as possible chemopreventive agents against intestinal tumorigenesis in the Min mouse. Nutr Cancer. 2006. 54:79–83.

21. Park JH, Oh SM, Lim SS, Lee YS, Shin HK, Oh YS, Choe NH, Park JH, Kim JK. Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochem Biophys Res Commun. 2006. 351:146–152.

22. Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders. N Engl J Med. 1979. 301:77–85.

23. Rogers GS, Gilchrest BA. The senile epidermis:environmental influences on skin aging and cutaneous carcinogenesis. Br J Dermatol. 1990. 122:55–60.

24. Saito Y, Shiga A, Yoshida Y, Furuhashi T, Fugita Y, Niki E. Effects of novel gaseous antioxidative system containing a Rosemary extract on the oxidation induced by nitrogen dioxide and ultraviolet radiation. Biosci Biotechnol Biochem. 2004. 68:781–786.

25. Skupien K, Oszmianski J, Kostrzewa-Nowak D, Tarasiuk J. In vitro antileukaemic activity of extracts from berry plantleaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006. 236:282–291.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download