Abstract
Purpose
For treating advanced non-small cell lung cancer (NSCLC), epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are known to be very effective in nonsmokers, women, Asian and person with EGFR mutations. The efficacy of EGFR-TKI was analyzed based on the radiologic studies and the serum levels of carcinoembryonic antigen (CEA) to evaluate whether serum CEA can be used as a predicative marker of the response to EGFR-TKI therapy.
Materials and Methods
Forty-one patients with NSCLC treated with gefitinib at Kosin Medical Center from January 2007 to August 2009 were the subjects of this study. We assayed the serum CEA levels before and after gefitinib therapy with concomitant assessments of the tumor response by serial chest X-ray and chest computer tomograms (CT).
Results
The median age of the patients was 62.6 years (range, 32∼77 years), 29 patients were women, 36 had adenocarcinoma (87.8%) and the baseline serum CEA was equal or above 5 ng/mL in 31 patients (75.6%). These 31 patients were more responsive to the gefitinib therapy (p=0.021). The overall response rate of the patients was 51.2%, the median survival time was 21.9 months and the time to progression was 8.3 months. Among the 21 responding patients, the serum CEA was decreased after 2 months in 17 (80.9%), and among the 14 progressed patients, the serum CEA was increased in 12 (85.7%) (p=0.000).
Go to : 
References
1. Korean National Statistical Office. Annual report on the cause of death statistics. Daejon: Korean National Statistical Office;2005.
2. Fu XL, Zhu XZ, Shi DR, et al. Study of prognostic predictors for nonsmall cell lung cancer. Lung Cancer. 1999; 23:143–152.

3. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in nonsmall cell lung cancer: a metaanalysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995; 311:899–909.
4. Raben D, Helfrich BA, Chan D, et al. ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combination with radiation and chemotherapy as a new therapeutic strategy in nonsmall cell lung cancer. Semin Oncol. 2002; 29(1 Suppl 4):37–46.

5. Kim YT, Kim C, Sohn JH, et al. The effect of ZD 1839 (IressaⓇ) in the treatment of refractory non small cell lung cancer. Cancer Res Treat. 2003; 35:502–506.
6. Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced nonsmall-cell lung cancer. J Clin Oncol. 2004; 22:1103–1109.

7. Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate antiapoptotic pathways. Science. 2004; 305:1163–1167.
8. Benchimol S, Fuks A, Jothy S, et al. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989; 57:327–334.

9. Hammarströ m S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999; 9:67–81.
10. Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997; 137:939–952.

11. Haam SJ, Kim GD, Cho SH, et al. Clinical effectiveness of tumor markers (CEA, NSE, Cyfra 21–1) in completely resected nonsmall cell lung cancer. J Lung Cancer. 2006; 5:75–83.

12. Jou SS, Park JS, Kim YT, et al. The CT findings and the peak SUV on PET/CT according to the levels of Cyfra 21–1 and CEA in patients with nonsmall cell lung carcinoma. J Korean Soc Radiol. 2009; 61:227–235.

13. Ardizzoni A, Cafferata MA, Tiseo M, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006; 107:2842–2849.

14. Strimpakos AS, Petkar I, Mikropoulos C, et al. The incidence and prognostic significance of carcinoembryonic antigen (CEA) flare in patients with metastatic colorectal cancer (mCRC) receiving first-line chemotherapy. Paper presented at: 2009 Gastrointestinal Cancers Symposium; January 15–17. 2009. San Francisco, CA, USA.
Go to : 
Figures and Tables
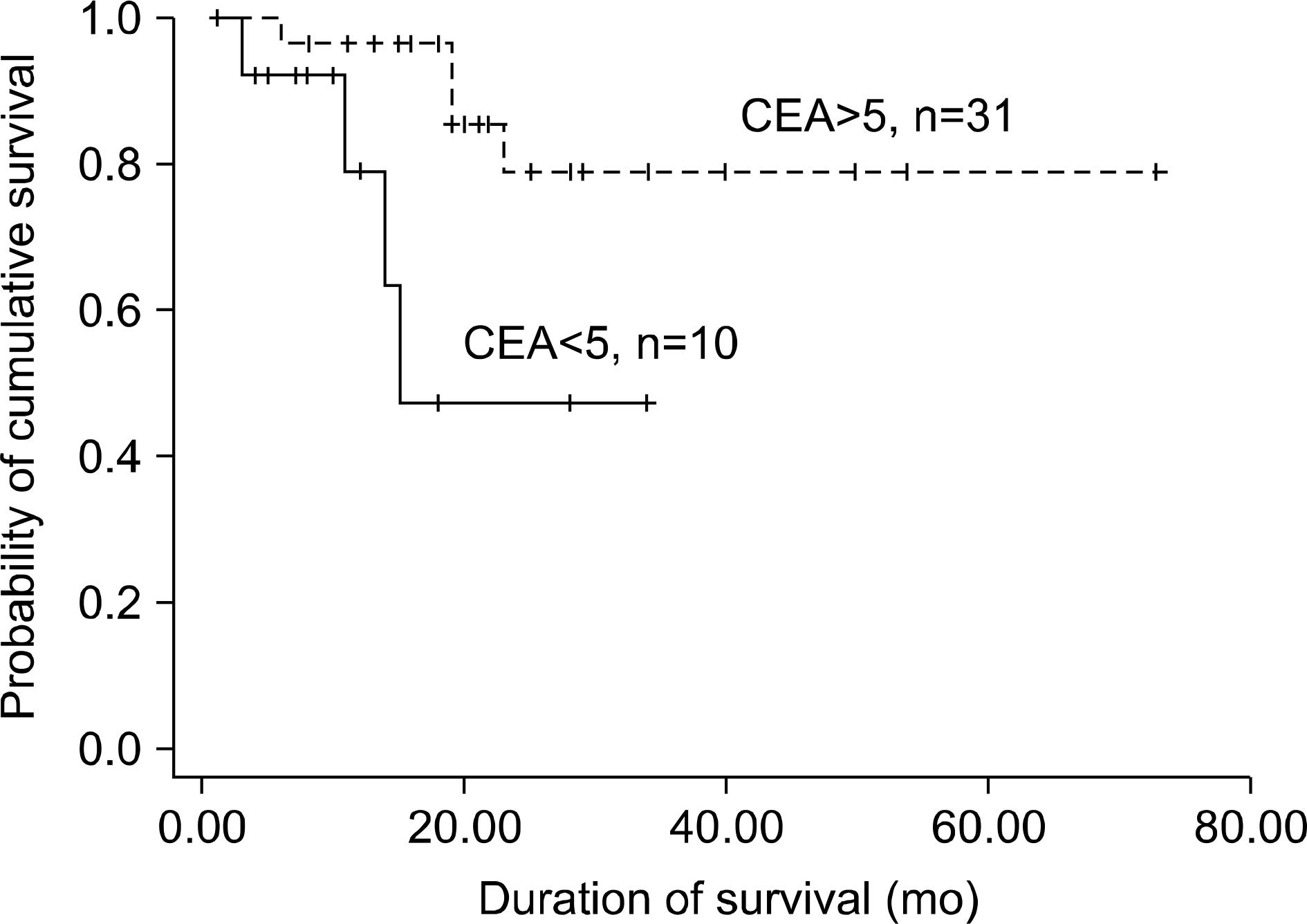 | Fig. 1.Survival of the patients according to the initial carcinoembryonic antigen (CEA) level (p<0.01). |
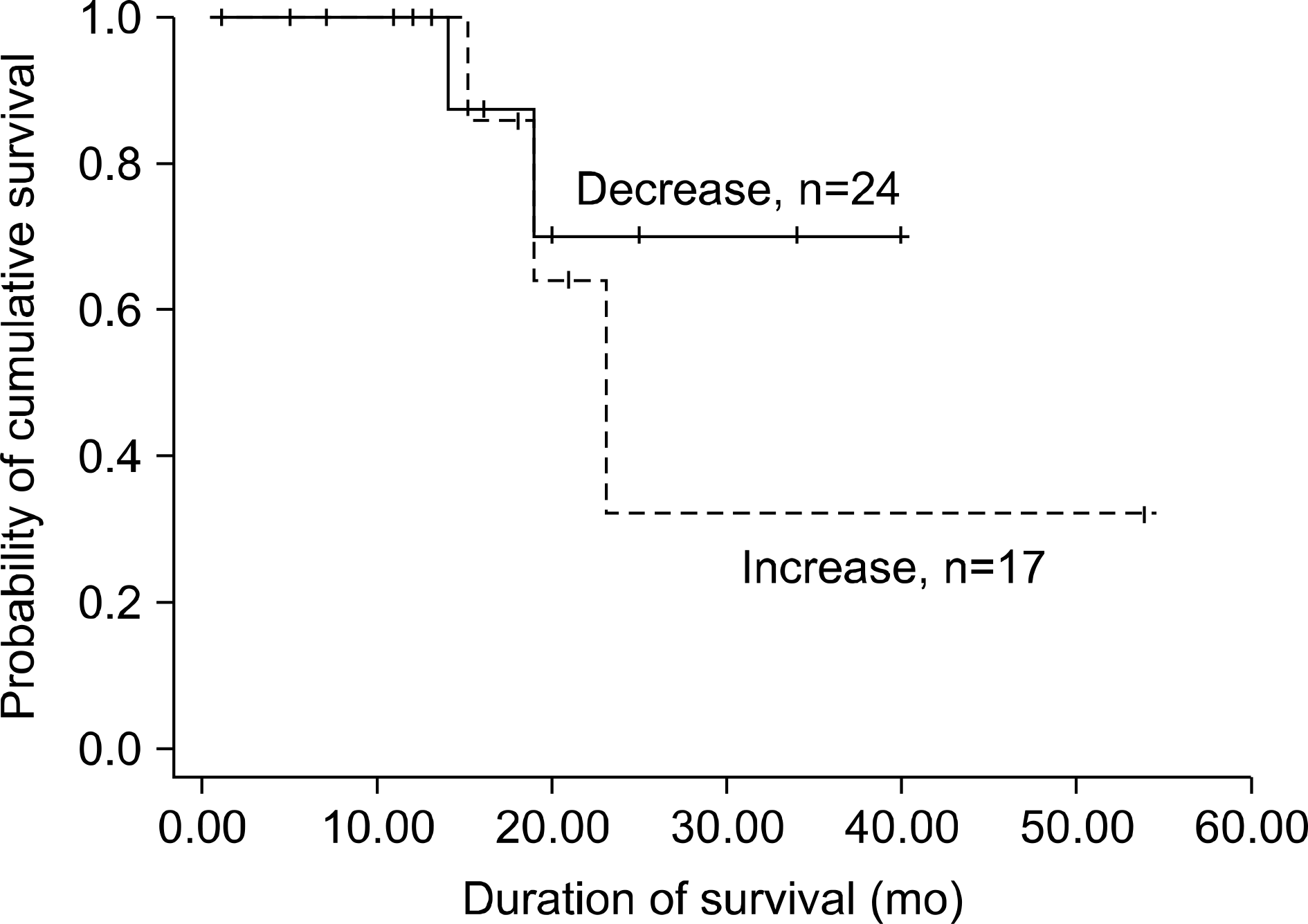 | Fig. 2.Survival of the patients according to the carcinoembryonic antigen (CEA) changes after gefitinib treatment (p <0.001). |
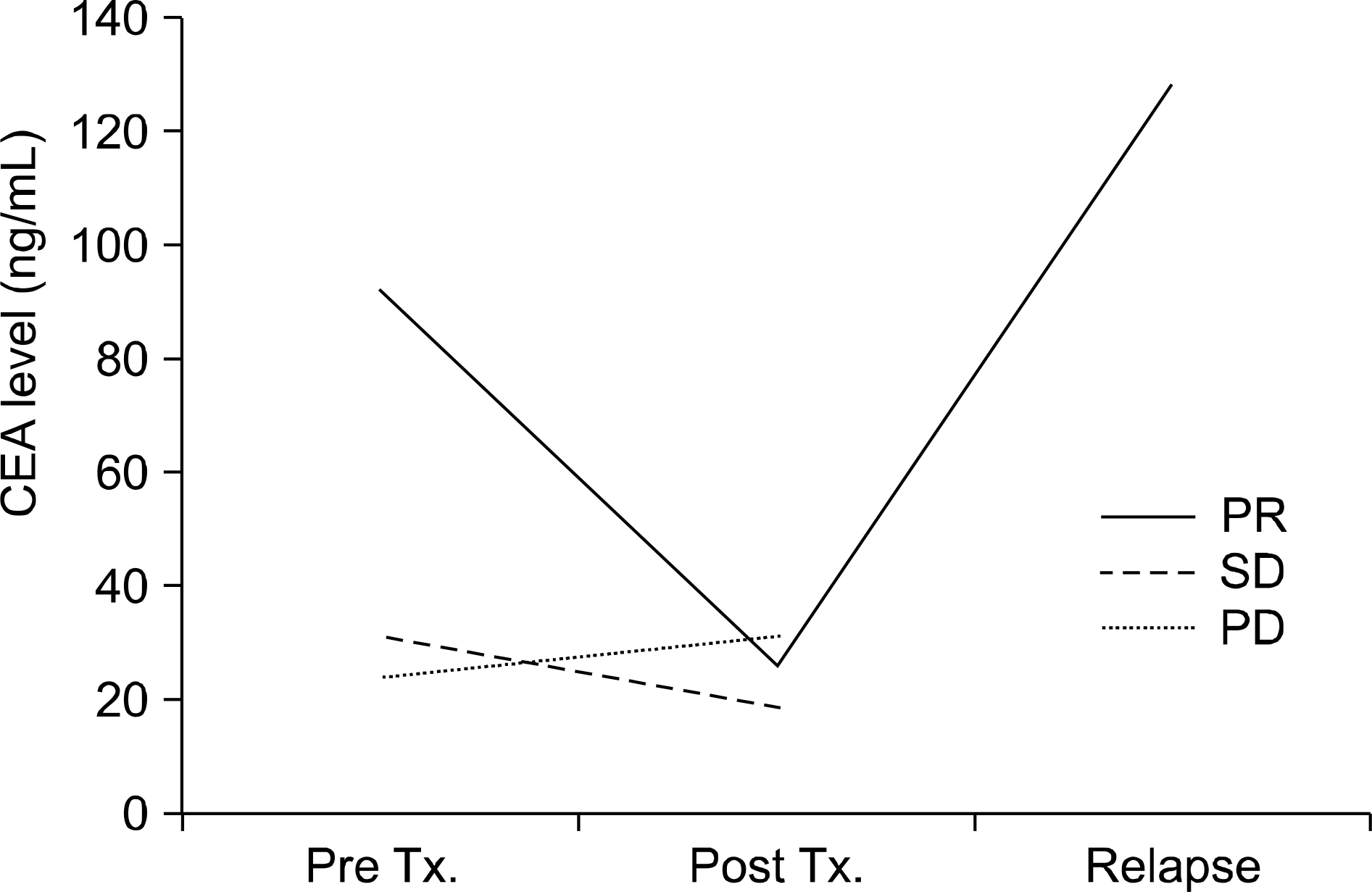 | Fig. 4.Changes in the carcinoembryonic antigen level according to the response to gefitinib. PR: partial response, SD: stable disease, PD: progressive disease. |
Table 1.
Clinicopathological Characteristics of the 41 Patients Treated with Gefitinib
Table 2.
Comparison of the Clinicopathological Characteristics of the Patients with a Partial Response, Stable Disease, and Progressive Disease after Gefitinib Treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download