Abstract
The advent of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib and erlotinib has opened a new horizon for the therapeutic options for patients with advanced lung cancer. Treatment paradigms are shifting from cytotoxic chemotherapies to molecular-based targeted therapies. The discovery of somatic mutations in the exons 18 to 21 of the tyrosine kinase (TK) domain of EGFR has revolutionized the understanding of EGFR in lung carcinogenesis and this has opened a new era for the importance of predictive biomarkers to select the treatment of choice and for personalized therapy for lung cancer. Three important EGFR assays are used and these include mutational analysis, fluorescence in situ hybridization and immunohistochemistry. EGFR mutation study seems to be the most important biomarker to predict the response to EGFR-TKI, yet technical standardization for analyzing the status of EGFR mutation is the key factor. Therefore, it is important to standardize the approach and decide which assays are best to predict a patient's response to targeted therapies. It is also essential to determine the most cost-effective way to integrate EGFR molecular assays into clinical practice. This review will address the practical aspects of each of the currently proposed assays that have focused on EFGR mutational analysis and also the other important molecular markers such as k-ras mutation, the EML4-ALK fusion oncogene, ERCC1 and RRM1.
Go to : 
References
1. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.

2. Paez JG, Jä nne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.
3. Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and nonsmall-cell lung cancer: current knowledge and future directions. J Clin Oncol. 2005; 23:2556–2568.

4. Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in nonsmall-cell lung cancer. J Natl Cancer Inst. 2005; 97:643–655.

5. Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008; 26:4268–4275.
6. Mano H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci. 2008; 99:2349–2355.
7. Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in nonsmall-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006; 355:983–991.

8. Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in nonsmall-cell lung cancer. Lancet Oncol. 2009; 10:1001–1010.

10. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carbo-platin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957.

11. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007; 7:169–181.

12. Chou TY, Chiu CH, Li LH, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with nonsmall cell lung cancer. Clin Cancer Res. 2005; 11:3750–3757.

13. Cortes-Funes H, Gomez C, Rosell R, et al. Epidermal growth factor receptor activating mutations in Spanish gefitinib-treated nonsmall-cell lung cancer patients. Ann Oncol. 2005; 16:1081–1086.

14. Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in nonsmall cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006; 12:3908–3914.

15. Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006; 12:6494–6501.

16. Pao W, Ladanyi M. Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res. 2007; 13:4954–4955.
17. Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T. A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn. 2006; 8:335–341.

18. Tanaka T, Nagai Y, Miyazawa H, et al. Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for nonsmall cell lung cancers. Cancer Sci. 2007; 98:246–252.

19. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with nonsmall-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128.

20. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–1043.
21. Engelman JA, Jä nne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in nonsmall cell lung cancer. Clin Cancer Res. 2008; 14:2895–2899.
22. Belani CP. The role of irreversible EGFR inhibitors in the treatment of nonsmall cell lung cancer: overcoming resistance to reversible EGFR inhibitors. Cancer Invest. 2010; 28:413–423.

23. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008; 359:366–377.
24. Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005; 23:6838–6845.

25. Gallegos Ruiz MI, Floor K, Vos W, et al. Epidermal growth factor receptor (EGFR) gene copy number detection in nonsmall-cell lung cancer: a comparison of fluorescence in situ hybridization and chromogenic in situ hybridization. Histopathology. 2007; 51:631–637.

26. Sholl LM, John Iafrate A, Chou YP, et al. Validation of chromogenic in situ hybridization for detection of EGFR copy number amplification in nonsmall cell lung carcinoma. Mod Pathol. 2007; 20:1028–1035.

27. Suzuki S, Dobashi Y, Sakurai H, Nishikawa K, Hanawa M, Ooi A. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: an immunohistochemical and fluorescence in situ hybridization study. Cancer. 2005; 103:1265–1273.
28. Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced nonsmall-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009; 373:1525–1531.

29. Brevet M, Arcila M, Ladanyi M. Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn. 2010; 12:169–176.

30. Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with nonsmall-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005; 23:5900–5909.

31. Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of nonsmall-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010; 17:889–897.

32. Bang Y, Kwak EL, Shaw DR, et al. Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with nonsmall cell lung cancer (NSCLC). Proc Am Soc Clin Oncol. 2010; 28:A3.

33. Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010; 16:1561–1571.

34. Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007; 356:800–808.
Go to : 
Figures and Tables
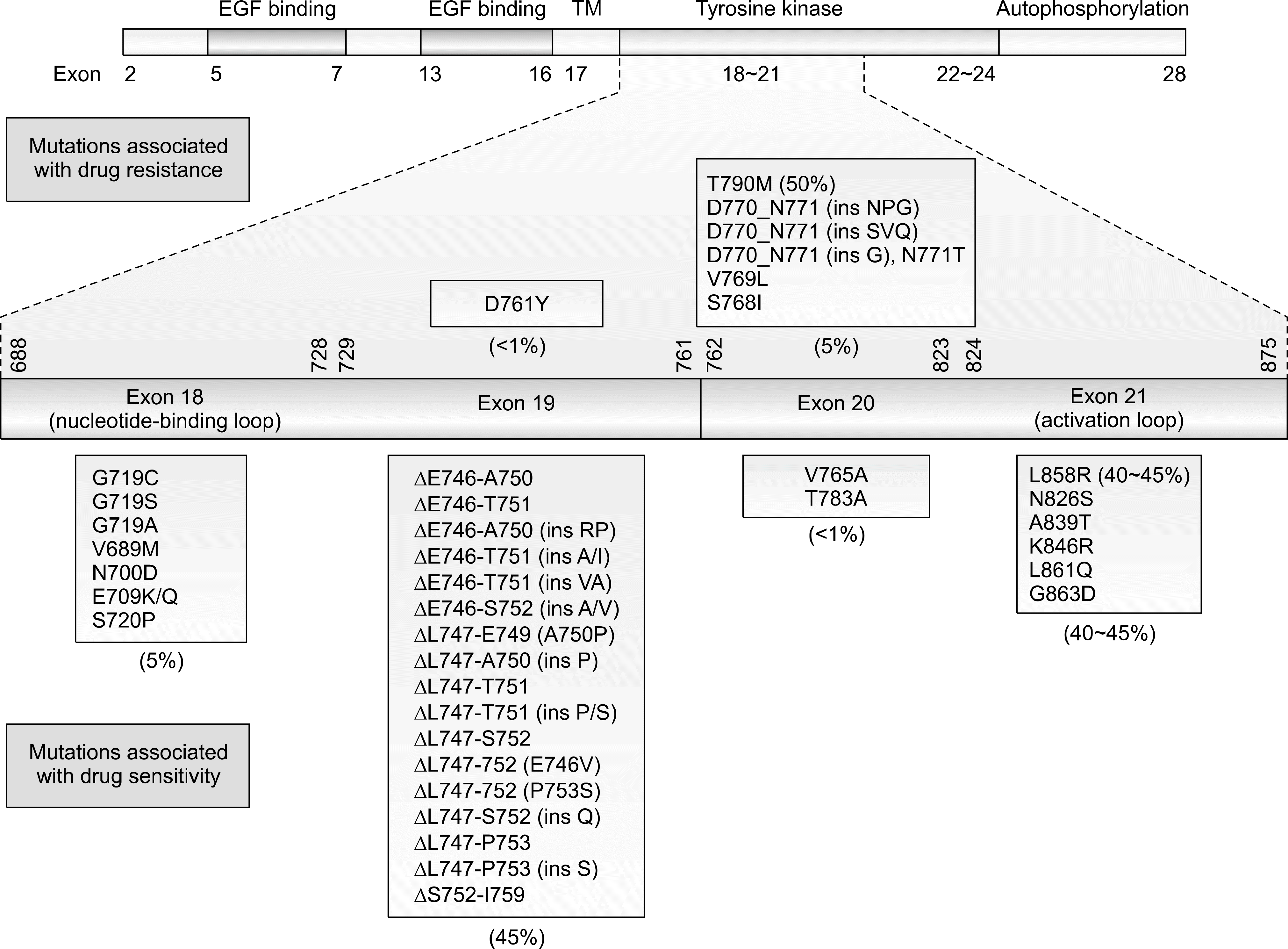 | Fig. 1.Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs)-sensitizing mutations in non small cell lung cancer. Adapted from Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer (Adapted from Sharma SV, et al. Nat Rev Cancer 2007;7:169–181) (11). |
Table 1.
Technical Comparisons among the Methods to Analyze Epidermal Growth Factor Receptor Mutations




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download