Abstract
Purpose
The recent introduction of targeted therapy for non-small cell lung cancer (NSCLC) has changed the paradigm of lung cancer chemotherapy. However, only a small portion of NSCLC patients received the benefit of these new drugs. A proteasome inhibitor (bortezomib) and a histone deacetylase inhibitor (SAHA) were approved for clinical use for treating some hematologic malignancies. In this study, we investigated the combination treatment of bortezomib and SAHA in NSCLC cell lines.
Materials and Methods
The combined effects of bortezomib and SAHA on lung cancer cell lines were measured by Calcusyn software. Induction of apoptosis was examined by performing an Annexin V assay. Generation of reactive oxygen species (ROS) and protection by N-acetylcysteine were measured by flow cytometry after staining with DCFH-DA. The effect of the combination of drugs on apoptosis and autophagy was investigated by Western blot assay.
Results
Strong synergism was found for the combination of bortezomib and SAHA. The synergistic interaction was mediated by strong apoptosis. Increased ROS generation was partly responsible for the induction of apoptosis, and this was suppressed by the ROS scavenger N-acetylcysteine. Combined treatment induced the strong activation of caspase-3 and the breakdown of the antiapoptotic molecule Bcl-2. Furthermore, increased breakdown of beclin-1, which is known to be an autophagic molecule, was also found.
Conclusion
Combination therapy with bortezomib and SAHA showed a strong synergistic antitumor effect on human lung cancer cell lines. Enhanced induction of apoptosis was a responsible mechanism, and this was partly mediated by ROS generation. Further studies are warranted for determining the role of apoptosis and autophagy for this combination therapy.
References
1. Ludwig H, Khayat D, Giaccone G, et al. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer. 2005; 104:1794–1807.

2. Genin E, Reboud-Ravaux M, Vidal J. Proteasome inhibitors: recent advances and new perspectives in medicinal chemistry. Curr Top Med Chem. 2010; 10:232–256.

3. Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced nonsmall-cell lung cancer. J Clin Oncol. 2006; 24:5025–5033.

4. Somech R, Izraeli S, J Simon A. Histone deacetylase inhibitors–a new tool to treat cancer. Cancer Treat Rev. 2004; 30:461–472.
5. Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007; 109:31–39.

6. Komatsu N, Kawamata N, Takeuchi S, et al. SAHA, a HDAC inhibitor, has profound anti-growth activity against nonsmall cell lung cancer cells. Oncol Rep. 2006; 15:187–191.

7. Imre G, Gekeler V, Leja A, et al. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factoralpha receptor-1 down-regulation. Cancer Res. 2006; 66:5409–5418.
8. Yu C, Rahmani M, Conrad D, et al. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003; 102:3765–3774.

9. Emanuele S, Lauricella M, Carlisi D, et al. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis. 2007; 12:1327–1338.

10. Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008; 80:133–142.

11. Zhang QL, Wang L, Zhang YW, et al. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia. 2009; 23:1507–1514.

12. Heider U, Rademacher J, Lamottke B, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in cutaneous T cell lymphoma. Eur J Haematol. 2009; 82:440–449.

13. Alva AS, Gultekin SH, Baehrecke EH. Autophagy in human tumors: cell survival or death? Cell Death Differ. 2004; 11:1046–1048.

15. Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005; 1:66–74.

16. Li J, Liu R, Lei Y, et al. Proteomic analysis revealed association of aberrant ROS signaling with suberoylanilide hydroxamic acid-induced autophagy in Jurkat T-leukemia cells. Autophagy. 2010; 6:711–7124.

17. Belloni D, Veschini L, Foglieni C, et al. Bortezomib induces autophagic death in proliferating human endothelial cells. Exp Cell Res. 2010; 316:1010–1018.

18. Zhu K, Dunner K Jr, McConkey DJ. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010; 29:451–462.

19. Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004; 3:1124–1126.

Figures
Fig. 1.
Analysis of the drug interaction (SAHA and bortezomib) in the lung cancer cell lines. Calcusyn analysis (right column) demonstrated that SAHA and bortezomib showed strong synergistic cytotoxicity in two lung cancer cell lines (the combination indices for most drug combinations were below 0.7).
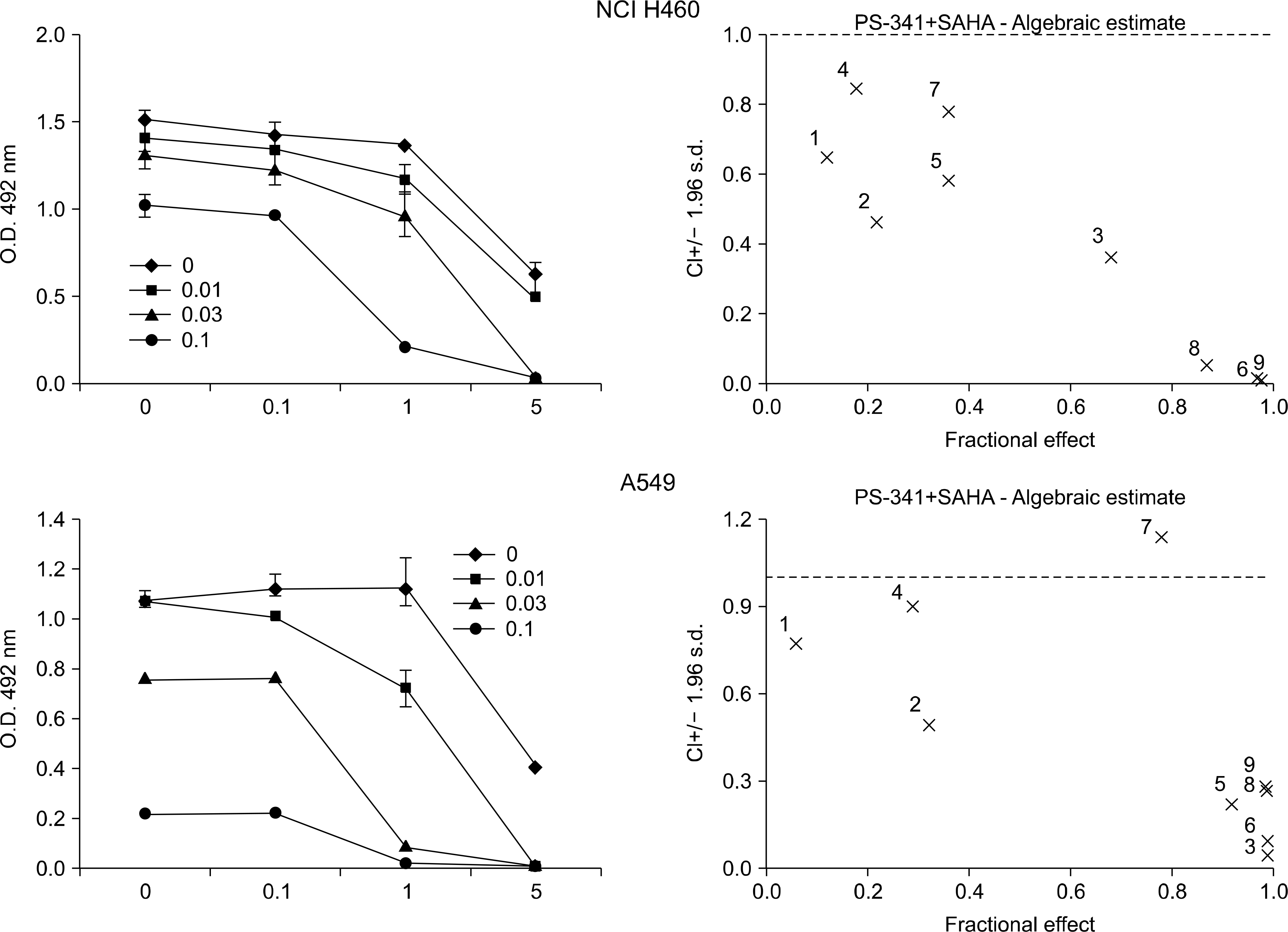
Fig. 2.
Increased apoptosis by combining SAHA and bortezomib in a lung cancer cell line. (A) NCI H460 cells were treated with SAHA (0, 1μ M) and bortezomib (0, 0.03, and 0.1μ M) for 48 hours and Annexin V assay was performed to measure the cell apoptosis. The cells treated with SAHA (1μ M) and bortezomib (0.1μ M) showed a high proportion of early apoptotic cells (24.96%) compared with the cells treated with SAHA (1μ M) alone (2.6%) and the cells treated with bortezomib (0.1μ M) alone (6.6%). (B) Data of the proportions of cells showing early apoptosis (lower right quadrant, LR) in three independent experiments (B, bortezomib; S, SAHA). All the experiments showed the same tendency of increased apoptosis by a combination of SAHA and bortezomib.
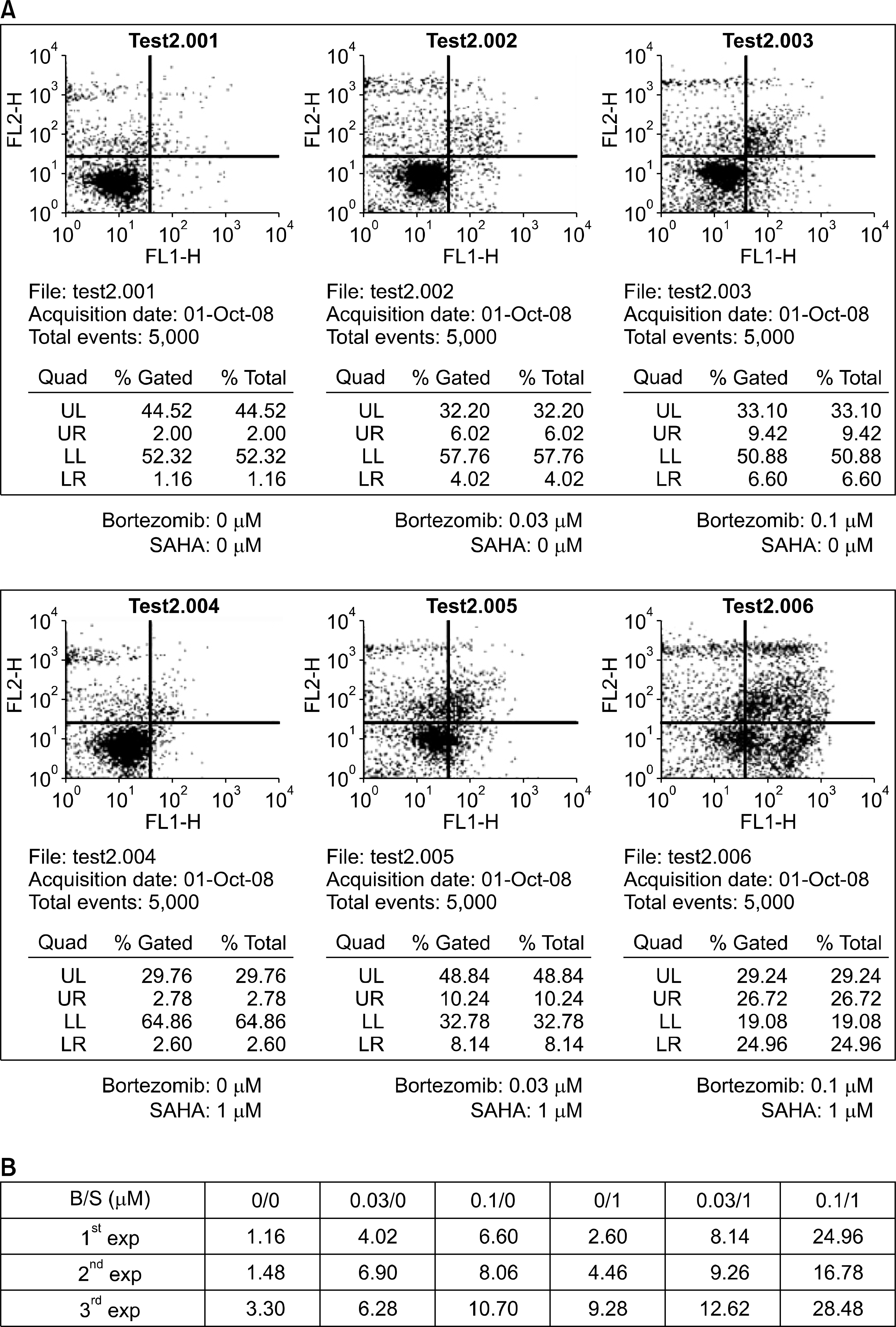
Fig. 3.
The apoptosis induced by SAHA and bortezomib was suppressed by N-acetylcysteine (NAc), which is a reactive oxygen species (ROS) scavenger. Pretreatment of lung cancer cells (A549 and NCI H460) with NAc (15 mM).
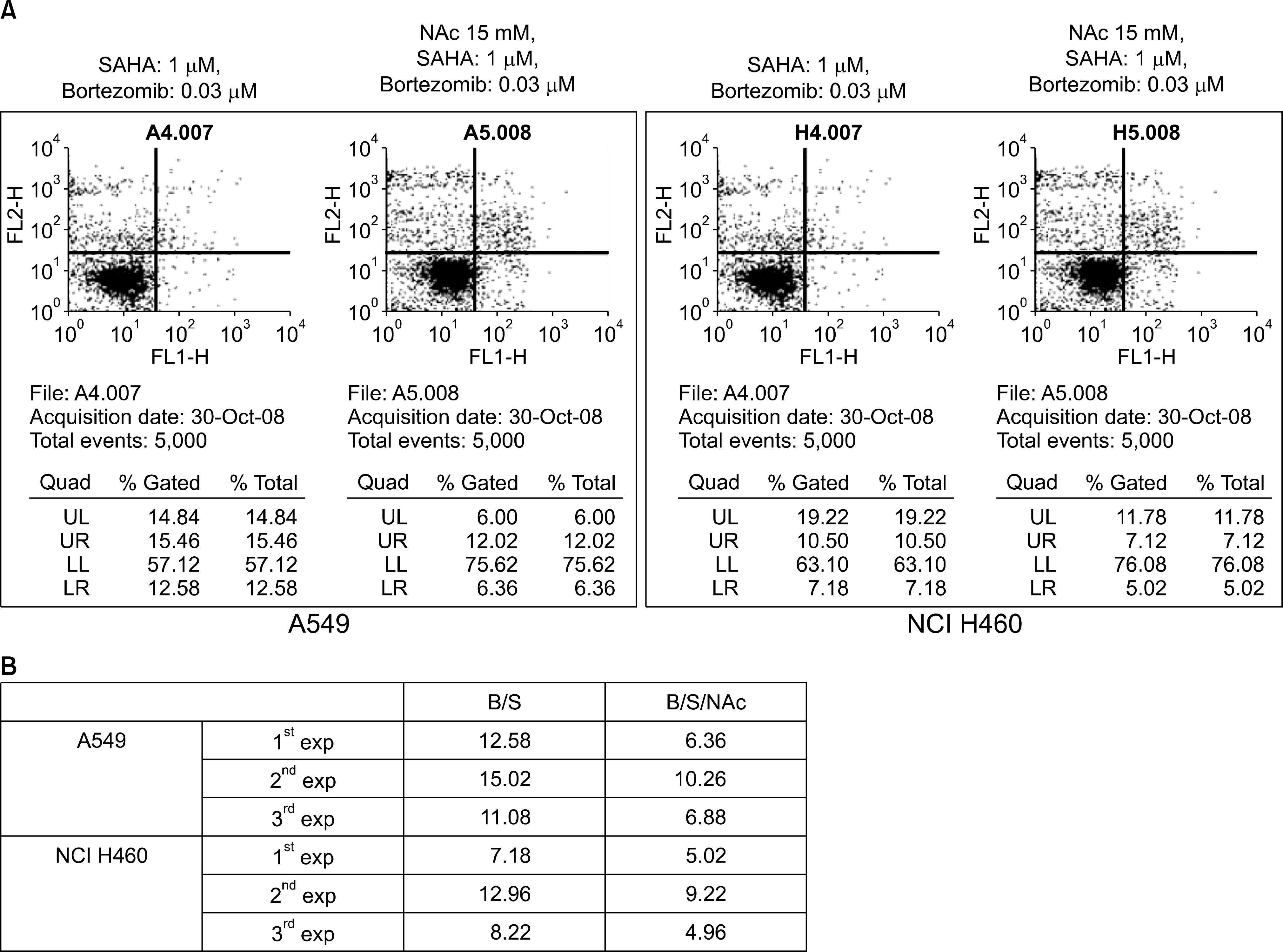
Fig. 4.
Increased reactive oxygen species (ROS) formation by combination of SAHA and bortezomib and reduced ROS formation by N-acetylcysteine (NAc). ROS was stained with DCFH-DA and ROS value was measured by flow cytometry. Three hour-pretreatment with NAc significantly reduced intracellular ROS formation induced by SAHA and bortezomib. Mean values of ROS in A549: 224.3 (native), 393.5 (bortezomib: 0.03μ M), 325.5 (SAHA: 1μ M), 459.5 (bortezomib+ SAHA) and 274.5 (bortezomib +SAHA+NAc). Mean values of ROS in NCI H460: 502.6 (native), 727.4 (bortezomib: 0.03μM), 535.1 (SAHA: 1μM), 1042.1 (bortezomib+ SAHA) and 745.8 (bortezomib+ SAHA+ NAc).
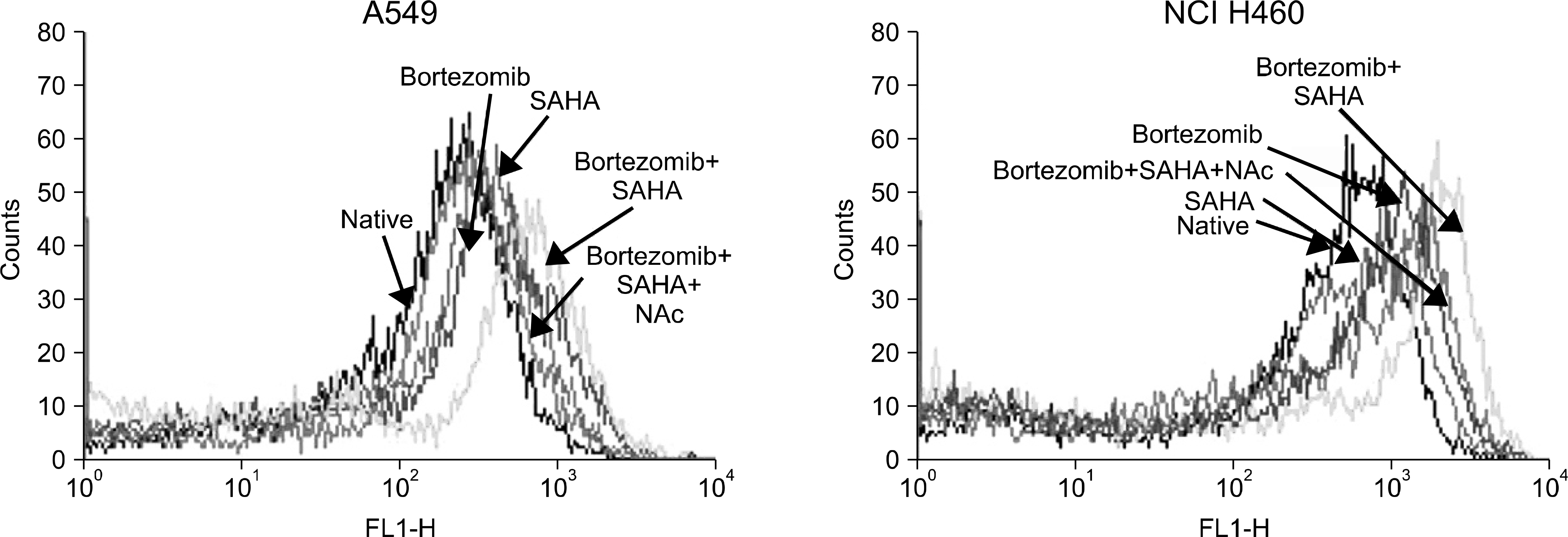
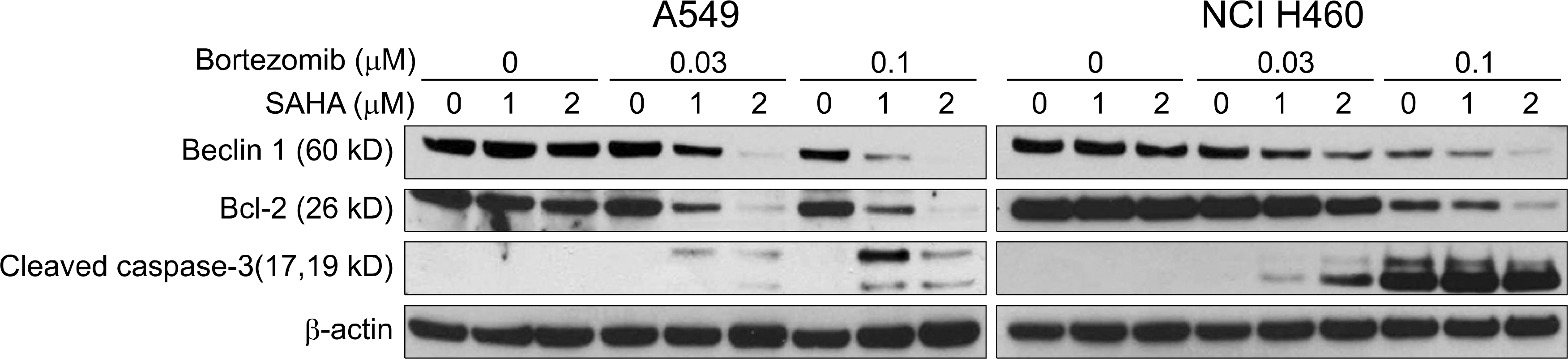
Fig. 5.
Analysis of apoptotic and autophagic pathway of lung cancer cells (A549 and NCI H460) treated with bortezomib and SAHA. Compared with single treatment of bortezomib or SAHA, combined treatment of bortezomib and SAHA induced enhanced cleavage of caspase 3 and breakdown of antiapoptotic molecule, Bcl-2. Furthermore, combined treatment of bortezomib and SAHA induced breakdown of beclin-1 known as autophagic molecule. Details are described in the text.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download