Abstract
Purpose
Epidermal growth factor (EGF) is known to cause cellular proliferation, differentiation and survival. However, there are a few articles that have reported on the cell killing effect of EGF. We evaluated the effects of EGF on the survival of some lung cancer cell lines and we tried to determine the mechanism of action.
Materials and Methods
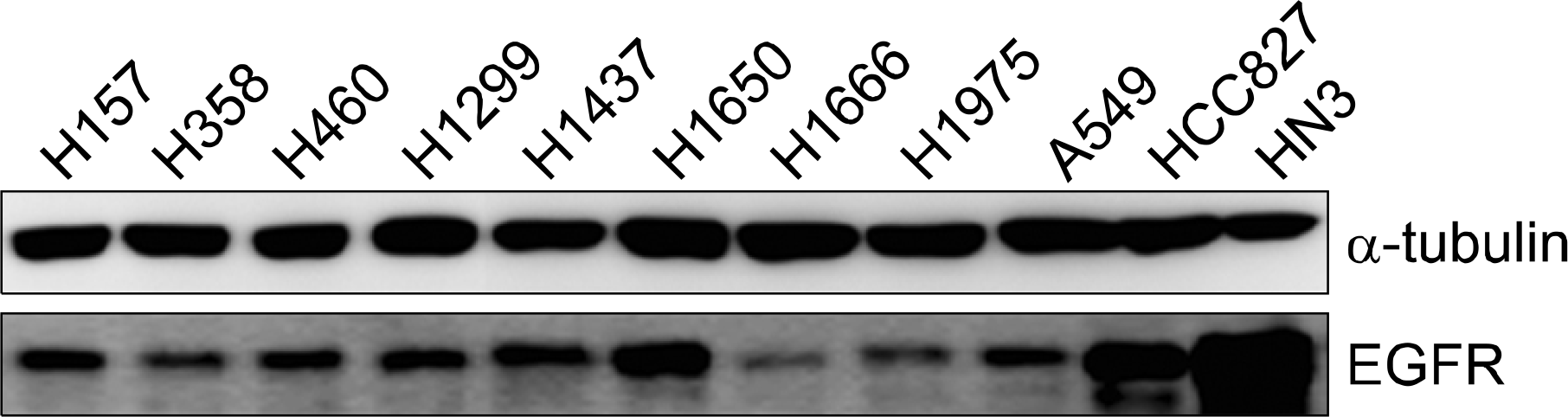
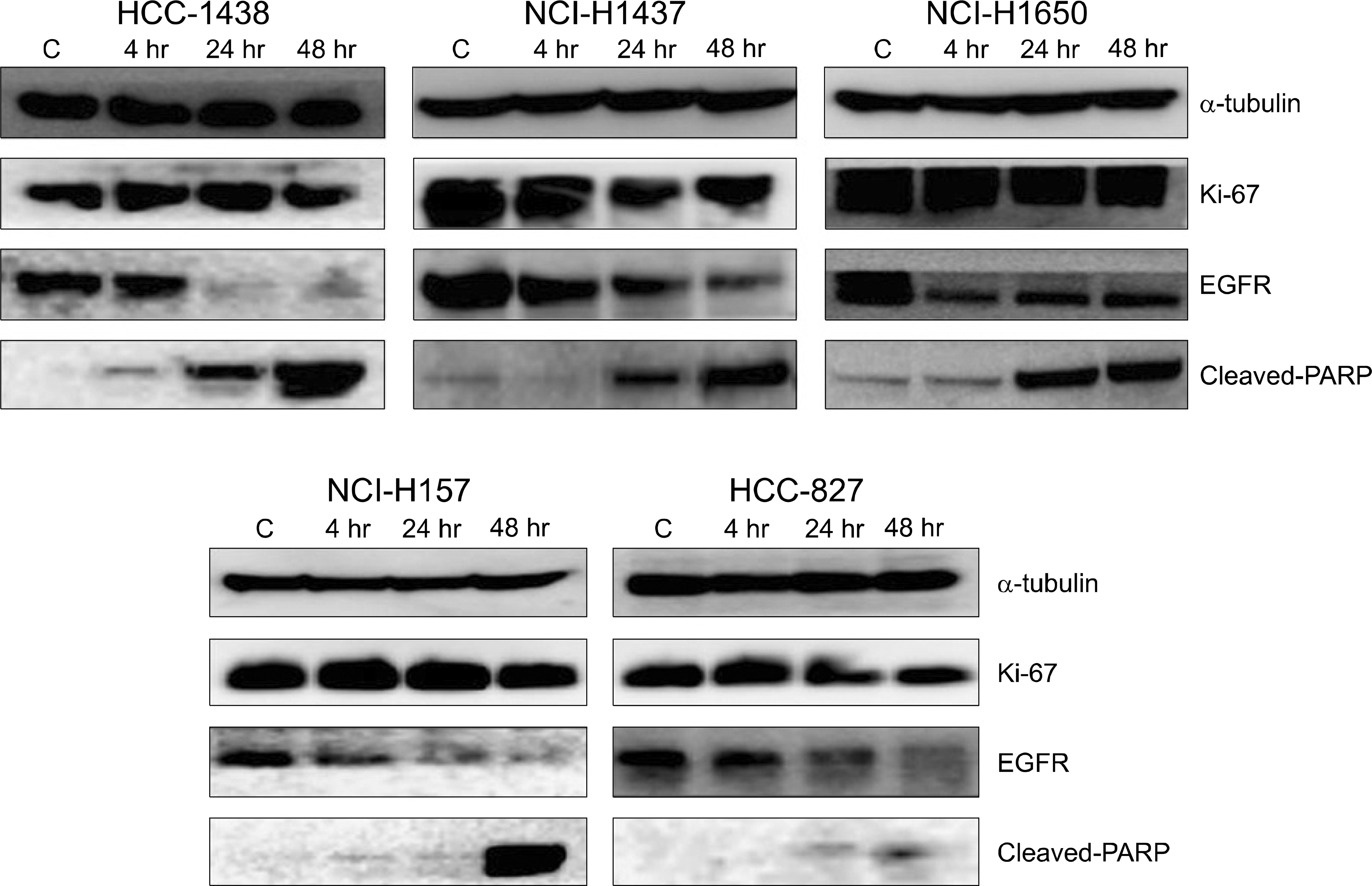
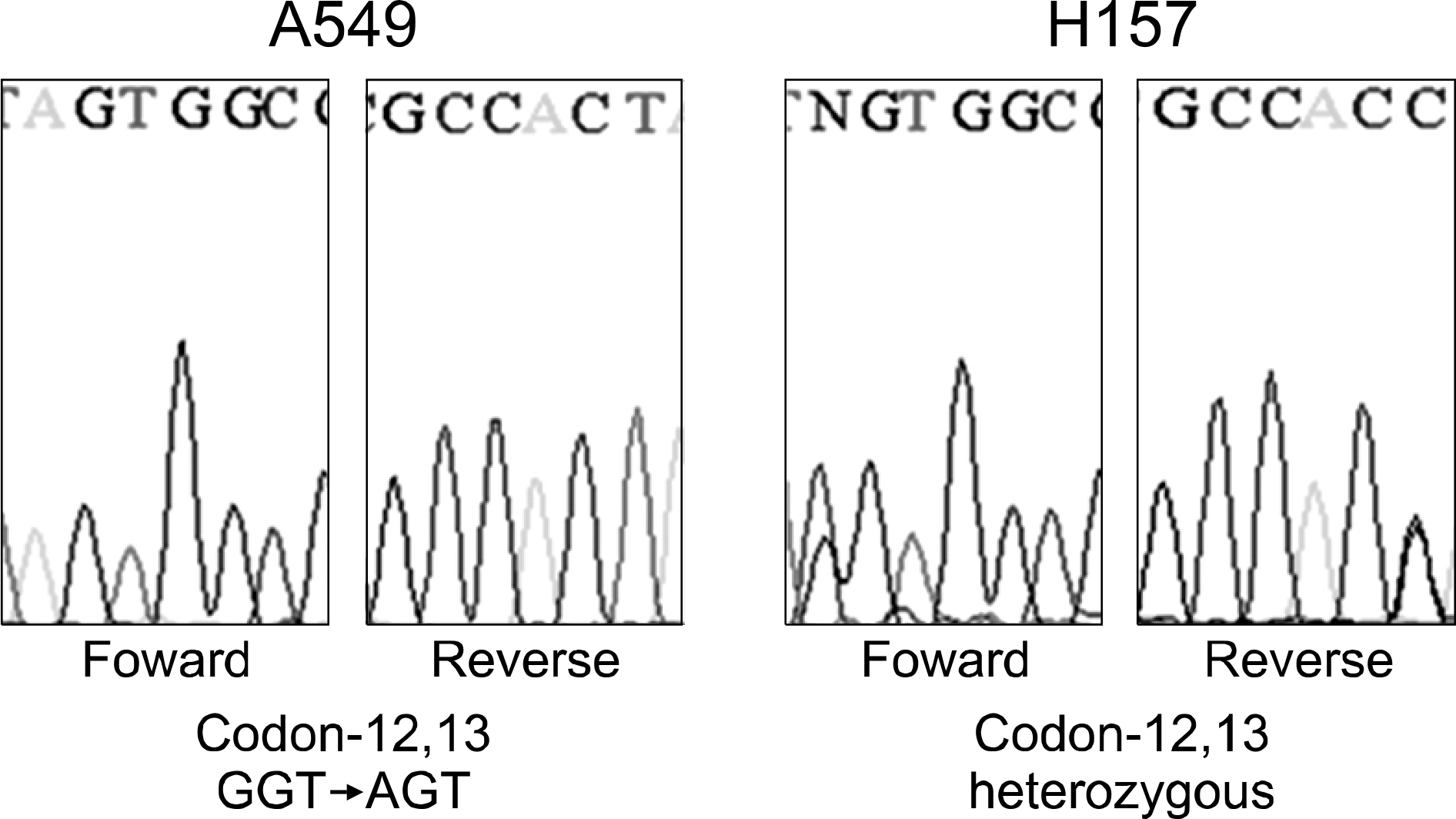
We examined various lung cancer cell lines. The cultured cells were exposed to radiation alone (0, 2, 5, and 10 Gy), EGF alone (0∼1,000 nM) or a combination of radiation and EGF (10 nM). Survival was measured using a clonogenic assay and the expressions of epidermal growth factor receptor (EGFR), Ki-67 and cleaved-PARP were detected by western blot analysis. K-ras mutations were detected using polymerase chain reaction and sequencing.
Results
Treatment of EGF induced cell death in the lung cancer cell lines. The addition of EGF (10 nM) to radiation (0, 2, 5, and 10 Gy) resulted in an increased cell killing effect of radiation. The EGFR expression decreased with the addition of EGF. EGF increased the expression of cleaved-PARP, but it did not increase the expression of Ki-67. The effects of EGF were not correlated with EGFR mutation or K-ras mutation.
Go to : 
References
3. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the newborn animal. J Biol Chem. 1962; 237:1555–1562.

4. Servold SA. Growth factor impact on wound healing. Clin Podiatr Med Surg. 1991; 8:937–953.
5. Brown GL, Curtsinger L 3rd, Brightwell JR, et al. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986; 163:1319–1324.

6. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006; 56:394–398.

7. Lee SW, Jung KI, Kim YW, Jung HD, Kim HS, Hong JP. Effect of epidermal growth factor against radiotherapy-induced oral mucositis in rats. Int J Radiat Oncol Biol Phys. 2007; 67:1172–1178.

8. Mishima H, Nakamura M, Murakami J, Nishida T, Otori T. Transforming growth factor-beta modulates effects of epidermal growth factor on corneal epithelial cells. Curr Eye Res. 1992; 11:691–696.
9. Bianco R, Gelardi T, Damiano V, Ciardiello F, Tortora G. Rational bases for the development of EGFR inhibitors for cancer treatment. Int J Biochem Cell Biol. 2007; 39:1416–1431.

10. Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002; 7(Suppl 4):31–39.

11. Paez JG, Jä nne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.
12. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.

13. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004; 101:13306–13311.
14. Gill GN, Lazar CS. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981; 293:305–307.

15. Kamata N, Chida K, Rikimaru K, Horikoshi M, Enomoto S, Kuroki T. Growth-inhibitory effects of epidermal growth factor and overexpression of its receptors on human squamous cell carcinomas in culture. Cancer Res. 1986; 46:1648–1653.
16. MacLeod CL, Luk A, Castagnola J, Cronin M, Mendelsohn J. EGF induces cell cycle arrest of A431 human epidermoid carcinoma cells. J Cell Physiol. 1986; 127:175–182.

17. Krö ning R, Jones JA, Hom DK, et al. Enhancement of drug sensitivity of human malignancies by epidermal growth factor. Br J Cancer. 1995; 72:615–619.

18. Christen RD, Hom DK, Eastman A, Howell SB. Epidermal growth factor regulates the ability of human ovarian carcinoma cells to repair DNA damage. Proc Am Assoc Cancer Res. 1991; 32:430.
19. Kwok TT, Sutherland RM. Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J Natl Cancer Inst. 1989; 81:1020–1024.

20. Kwon EK, Lee SH, Kim KB, Wu HG, Lee SW. Differential effect of recombinant human EGF on proliferation and radiation survival of normal fibroblast and cancer cell lines. Open Transl Med J. 2009; 1:9–15.
21. Reynolds NA, Wagstaff AJ. Cetuximab: in the treatment of metastatic colorectal cancer. Drugs. 2004; 64:109–118.
22. Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and metaanalysis. Colorectal Cancer Collaborative Group. BMJ. 2000; 321:531–535.
23. Suzuki S, Dobashi Y, Sakurai H, Nishikawa K, Hanawa M, Ooi A. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas. An immunohistochemical and fluorescence in situ hybridization study. Cancer. 2005; 103:1265–1273.
24. Grudinkin PS, Zenin VV, Kropotov AV, Dorosh VN, Nikolsky NN. EGF-induced apoptosis in A431 cells is dependent on STAT1, but not on STAT3. Eur J Cell Biol. 2007; 86:591–603.

25. Filmus J, Trent JM, Pollak MN, Buick RN. Epidermal growth factor receptor gene-amplified MDA-468 breast cancer cell line and its nonamplified variants. Mol Cell Biol. 1987; 7:251–257.

26. Gulli LF, Palmer KC, Chen YQ, Reddy KB. Epidermal growth factor-induced apoptosis in A431 cells can be reversed by reducing the tyrosine kinase activity. Cell Growth Differ. 1996; 7:173–178.
27. Armstrong DK, Kaufmann SH, Ottaviano YL, et al. Epidermal growth factor-mediated apoptosis of MDA-MB-468 human breast cancer cells. Cancer Res. 1994; 54:5280–5283.
28. Brabyn CJ, Franks DJ, Kleine LP. Long-term exposure to epidermal growth factor results in apoptosis in T51B cells. Biochem Cell Biol. 1994; 72:429–438.

29. Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997; 64:33–42.

30. Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002; 7:321–328.
31. Scopa CD, Tsamandas AC, Zolota V, Kalofonos HP, Batistatou A, Vagianos C. Potential role of bcl-2 and ki-67 expression and apoptosis in colorectal carcinoma: a clinicopathologic study. Dig Dis Sci. 2003; 48:1990–1997.
32. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–346.

33. Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004; 64:8919–8923.
Go to : 
Figures
 | Fig. 1.The effect of epidermal growth factor (EGF) on the proliferation of the lung cancer cell lines. The cells were cultured for 24 hours prior to treatment with various concenrations (0, 0.01, 0.1, 1, 10, 100, and 1,000 nM) of EGF. The surviving cells were counted on day 10 using a clonogenic assay. |
 | Fig. 2.The effect of epidermal growth factor (EGF) on the proliferation of lung cancer cell lines. The cells were growth-arrested by incubation in medium containing 0.05% serum for 1 day. The cells were cultured for 12 days with various concenrations (0, 0.01, 0.1, 1, 10, 100, and 1,000 nM) of EGF. |
 | Fig. 3.Clonogenic survival of the lung cancer cells treated with epidermal growth factor (EGF) followed by irradiation with a range of radiation doses. Although the radiation sensitizing effect was minimal in the A549 cell line, the rest of lung cancer cell lines showed the radiation sensitizing effect of EGF. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download