Abstract
Purpose
Concurrent chemoradiotherapy (CCRT) is the standard treatment for locally advanced non-small cell lung cancer (NSCLC). Paclitaxel is an active agent against NSCLC and it has a radiosensitizing effect. We investigated the efficacy and toxicity of weekly paclitaxel administration along with concurrent radiotherapy for treating locally advanced and locally recurrent NSCLC.
Materials and Methods
Twenty-five previously untreated stage III or locally recurrent NSCLC patients received weekly paclitaxel (60 mg/m2) and concurrent radiotherapy. Chemotherapy was given on days 1, 8, 15 and 22. Concurrent radiotherapy at 1.5 Gy was given twice a day to a total dose of 54 Gy in 3.5 weeks. After the completion of CCRT, consolidation chemotherapy was delivered if possible.
Results
The overall response rate was 72% with one complete response and 17 partial responses. The median overall survival was 16 months with a 2 year survival rate and a 5 year survival rate of 38% and 24%, respectively. The rate of grade ≥3 radiation pneumonitis was 16% (4 patients) and 2 patients were died from the pneumonitis. The rate of grade 3 radiation esophagitis was 12% (3 patients) and the hematologic toxicities were not significant.
Go to : 
References
1. Bae JM, Won YJ, Jung KW, et al. Survival of Korean cancer patients diagnosed in 1995. Cancer Res Treat. 2002; 34:319–325.

2. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004; 350:379–392.

3. Bae KW, Song TH, Yang JY, et al. Concurrent chemoradiation with weekly paclitaxel in locally advanced nonsmall cell lung cancer. Tuberc Respir Dis. 2004; 57:351–357.

4. Cox JD, Pajak TF, Herskovic A, et al. Five-year survival after hyperfractionated radiation therapy for nonsmall-cell carcinoma of the lung (NSCLC): results of RTOG protocol 81–08. Am J Clin Oncol. 1991; 14:280–284.
5. Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III nonsmall-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996; 88:1210–1215.

6. Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy in inoperable nonsmall-cell lung cancer. N Engl J Med. 1992; 326:524–530.
7. Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, nonsmall-cell lung cancer: a meta-analysis. Ann Intern Med. 1996; 125:723–729.
8. Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III nonsmall-cell lung cancer. J Clin Oncol. 1999; 17:2692–2699.

9. Curran W Jr, Scott C, Langer C, et al. Phase III comparison of sequential vs. concurrent chemoradiation for patients with unresected stage III nonsmall cell lung cancer (NSCLC): initial report of Radiation Therapy Oncology Group (RTOG) 9410. Proc Am Soc Clin Oncol. 2000; 19:A1891.
10. Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced nonsmall-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Franç ais de Pneumo-Cancé rologie NPC 95–01 Study. J Clin Oncol. 2005; 23:5910–5917.
11. Caffo O. Radiosensitization with chemotherapeutic agents. Lung Cancer. 2001; 34(Suppl 4):S81–S90.

12. Yano T, Hara N, Ichinose Y, et al. Local recurrence after complete resection for nonsmall-cell carcinoma of the lung: significance of local control by radiation treatment. J Thorac Cardiovasc Surg. 1994; 107:8–12.

13. Shaw EG, Brindle JS, Creagan ET, Foote RL, Trastek VF, Buskirk SJ. Locally recurrent nonsmall-cell lung cancer after complete surgical resection. Mayo Clin Proc. 1992; 67:1129–1133.

14. Harpole DH Jr, Herndon JE 2nd, Young WG Jr, Wolfe WG, Sabiston DC Jr. Stage I nonsmall cell lung cancer: a multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995; 76:787–796.

16. Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992; 22:613–617.

17. Milas L, Hunter NR, Mason KA, Kurdoglu B, Peters LJ. Enhancement of tumor radioresponse of a murine mammary carcinoma by paclitaxel. Cancer Res. 1994; 54:3506–3510.
18. Milas L, Hunter NR, Mason KA, Milross CG, Saito Y, Peters LJ. Role of reoxygenation in induction of enhancement of tumor radioresponse by paclitaxel. Cancer Res. 1995; 55:3564–3568.
19. Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996; 88:1308–1314.

20. Kaplan EL, Meier P. Non-parametric estimations from incomplete observations. J Am Stat Assoc. 1958; 53:457–481.
21. Choy H, Safran H, Akerley W, Graziano SL, Bogart JA, Cole BF. Phase II trial of weekly paclitaxel and concurrent radiation therapy for locally advanced nonsmall cell lung cancer. Clin Cancer Res. 1998; 4:1931–1936.
22. Kirkbride P, Gelmon K, Eisenhauer E. Paclitaxel and concurrent radiotherapy in locally advanced nonsmall cell lung cancer: the Canadian experience. Semin Radiat Oncol. 1999; 9(2 Suppl 1):102–107.
23. Bae SH, Kim KC, Lee SA, et al. Efficacy of weekly paclitaxel and concurrent radiation therapy for locally advanced nonsmall cell lung cancer. Korean J Med. 2005; 69:379–386.
24. Kim S, Kim SW, Shim BY, et al. Treatment outcome of locally advanced nonsmall cell lung cancer patients who received concurrent chemoradiotherapy with weekly paclitaxel. J Korean Soc Ther Radiol Oncol. 2006; 24:230–236.
Go to : 
Figures and Tables
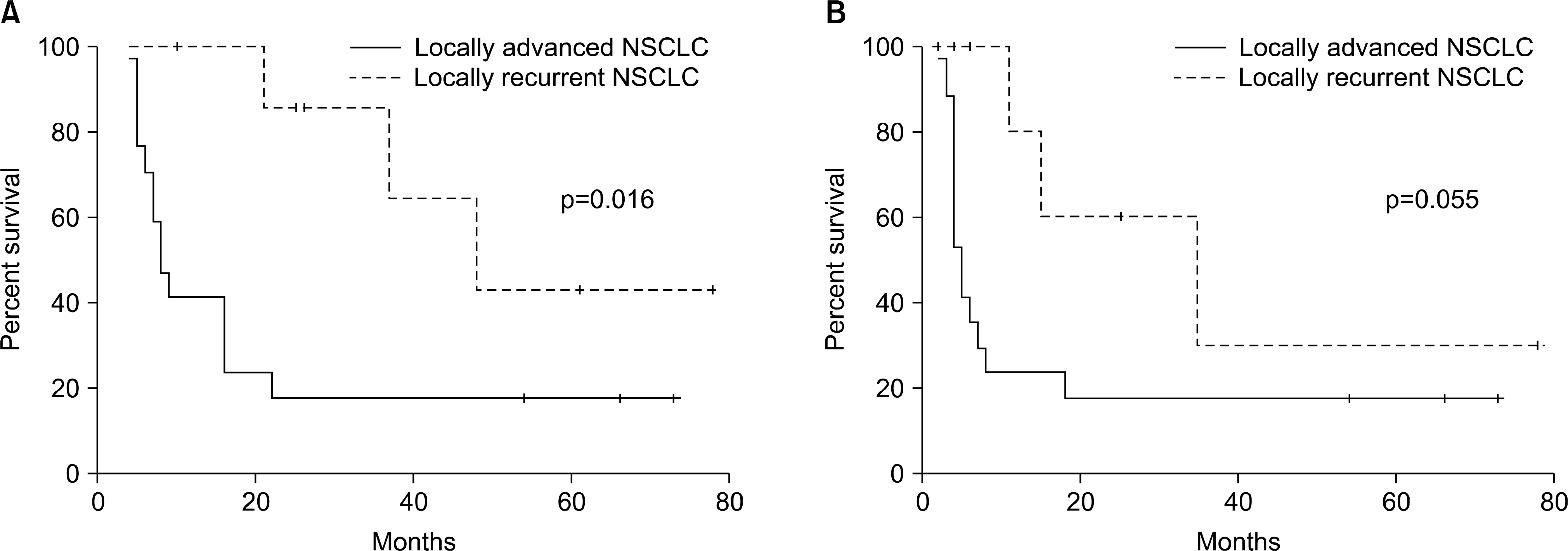 | Fig. 2.(A) Overall survival of locally advanced and locally recurrent NSCLC patients. (B) Progression-free survival of locally advanced and locally recurrent NSCLC patients. NSCLC: non-small cell lung cancer. |
Table 1.
Patient Characteristics




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download