Abstract
In this article, the modern concepts of computer-aided diagnosis (CAD), the methods of pulmonary nodule detection, and facts derived from the literature on the pulmonary nodule differential CAD are compiled in one source and described in some detail. Several issues in lung cancer (LC) epidemiology and early diagnosis are discussed. Analysis of research done so far shows evidence that various CAD systems can be successfully applied to chest radiographs, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). These modalities can serve as useful potential alternative tools available to practicing medical professionals performing routine diagnostics.
References
1. Wolbarst AB, Hendee WR. Evolving and experimental technologies in medical imaging. Radiology. 2006; 238:16–39.

2. Hendee WR, Ritenour ER. Medical imaging physics. 4th ed.New York: Wiley-Liss;2002.
3. Campadelli P, Casiraghi E, Artioli D. A fully automated method for lung nodule detection from posteroanterior chest radiographs. IEEE Trans Med Imaging. 2006; 25:1588–1603.

4. Hardie RC, Rogers SK, Wilson T, Rogers A. Performance analysis of a new computer aided detection system for identifying lung nodules on chest radiographs. Med Image Anal. 2008; 12:240–258.

5. Pereira C, Fernandes H, Mendonç a A, Campilho A. Detection of lung nodule candidates in chest radiographs. Lect Notes Comput Sci. 2007; 4478:170–177.

6. Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med. 2007; 357:2277–2284.
7. Doi K. Current status and future potential of computer-aided diagnosis in medical imaging. Br J Radiol. 2005; 78(Spec No 1):S3–S19.

8. Kahn CE Jr. Artificial intelligence in radiology: decision support systems. Radiographics. 1994; 14:849–861.

9. Rangayyan RM. Biomedical image analysis. Boca Raton, FL: CRC Press;2005.
11. Sluimer I, Schilham A, Prokop M, van Ginneken B. Computer analysis of computed tomography scans of the lung: a survey. IEEE Trans Med Imaging. 2006; 25:385–405.

12. Sluimer I, Prokop M, van Ginneken B. Toward automated segmentation of the pathological lung in CT. IEEE Trans Med Imaging. 2005; 24:1025–1038.

13. van Ginneken B, ter Haar Romeny BM, Viergever MA. Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging. 2001; 20:1228–1241.

14. Li Q. Recent progress in computer-aided diagnosis of lung nodules on thin-section CT. Comput Med Imaging Graph. 2007; 31:248–257.

15. Armato SG 3rd, McLennan G, McNitt-Gray MF, et al. Lung image database consortium: developing a resource for the medical imaging research community. Radiology. 2004; 232:739–748.

16. Aberle DR, Chiles C, Gatsonis C, et al. Imaging and cancer: research strategy of the American College of Radiology Imaging Network. Radiology. 2005; 235:741–751.

17. Rao D, Debb S, Blitz D, Choi SW, Cella D. Racial/Ethnic differences in the health-related quality of life of cancer patients. J Pain Symptom Manage. 2008; 36:488–496.

18. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007; 75:56–63.
20. Field JK, Youngson JH. The Liverpool Lung Project: a molecular epidemiological study of early lung cancer detection. Eur Respir J. 2002; 20:464–479.

21. Tang J, Rangayyan R, Yao J, Yang Y. Digital image processing and pattern recognition techniques for the detection of cancer. Pattern Recognit. 2008; 42:1015–1016.

23. Azzoli CG. Dx/Rx: Lung Cancer. Sudbury, MA: Jones and Bartlett;2006.
24. Feig B, Berger D, Fuhrman G, et al. The M.D. Anderson surgical oncology handbook. 4th ed.Philadelphia, PA: Lippincott Williams & Wilkins;2006.
25. Dargan EL. The enigma of the solitary pulmonary nodule. J Natl Med Assoc. 1973; 65:101–103. passim.
26. Joynt GH, Vassal KP. Solitary pulmonary nodule. Can Med Assoc J. 1959; 81:78–81.
27. Steele JD, Rigler LG. The solitary pulmonary nodule. Springfield: Charles C. Thomas;1964.
28. Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007; 132:108S–130S.
29. Kowalewski J. Subcentimeter pulmonary nodule: diagnostic and therapeutic problems. Pol Merkur Lekarski. 2008; 25:368–373.
30. Chen TM, Gould M. Evaluation of patients with small, subcentimeter nodules. Semin Respir Crit Care Med. 2008; 29:241–247.

31. Jin SM, Choi SH, Yoo CG, et al. Small solid noncalcified pulmonary nodules detected by screening chest computed tomography. Respir Med. 2007; 101:1880–1884.

32. Ketchedjian A, Daly B, Landreneau R, Fernando H. Sublobar resection for the subcentimeter pulmonary nodule. Semin Thorac Cardiovasc Surg. 2005; 17:128–133.

33. Altorki NK, Yankelevitz DF, Vazquez MF, Kramer A, Henschke CI. Bronchioloalveolar carcinoma in small pulmonary nodules: clinical relevance. Semin Thorac Cardiovasc Surg. 2005; 17:123–127.

34. Daniel TM. A proposed diagnostic approach to the patient with the subcentimeter pulmonary nodule: techniques that facilitate video-assisted thoracic surgery excision. Semin Thorac Cardiovasc Surg. 2005; 17:115–122.

35. Kernstine KH, Grannis FW Jr, Rotter AJ. Is there a role for PET in the evaluation of subcentimeter pulmonary nodules? Semin Thorac Cardiovasc Surg. 2005; 17:110–114.

36. Tsuchiya R. Implication of the CT characteristics of subcentimeter pulmonary nodules. Semin Thorac Cardiovasc Surg. 2005; 17:107–109.

37. Miller DL. Management of the subcentimeter pulmonary nodule. Semin Thorac Cardiovasc Surg. 2002; 14:281–285.

38. Gurney JW. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part I. Theory. Radiology. 1993; 186:405–413.

39. Gurney JW. Missed lung cancer at CT: imaging findings in nine patients. Radiology. 1996; 199:117–122.

40. Dolejsi M, Kybic J. Detection of pulmonary nodules in CT Scans. In: Jan J, Kozumplií k J, Provaznií k I, editors. Analysis of biomedical signals and images: proceedings of 18th International EURASIP Conference Biosignal 2006. Brno: Univ. of Technology;. 2006. 251–253.
41. Dolejsi M, Kybic J. Automatic two-step detection of pulmonary nodules. Proc Soc Photo Opt Instrum Eng. 2007; 6514:65143J.
42. Parr LH. Coin lesions of the lung. J Natl Med Assoc. 1969; 61:153–157.
43. Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003; 348:2535–2542.
44. Wilmore DW. American College of Surgeons. ACS surgery: principles and practice. New York: WebMD Corp.;2001.
45. Schwartz SI, Brunicardi FC. Schwartz's manual of surgery. 8th ed.New York: McGraw-Hill Medical Publishing Division;2006.
46. Hansen HH. Textbook of lung cancer. 2nd ed.London: Informa Healthcare;2008.
47. Sellke FW, del Nido PJ, Swanson SJ, et al. Sabiston & Spencer surgery of the chest. 7th ed.Philadelphia, PA: Elsevier Saunders;2005.
48. Dä hnert W. Radiology review manual. 6th ed.Philadelphia, PA: Lippincott Williams Wilkins;2007.
49. Webb WR. Radiologic evaluation of the solitary pulmonary nodule. AJR Am J Roentgenol. 1990; 154:701–708.

51. Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008; 246:772–782.

52. White CS, Salis AI, Meyer CA. Missed lung cancer on chest radiography and computed tomography: imaging and medico-legal issues. J Thorac Imaging. 1999; 14:63–68.
53. Armato SG 3rd, Li F, Giger ML, MacMahon H, Sone S, Doi K. Lung cancer: performance of automated lung nodule detection applied to cancers missed in a CT screening program. Radiology. 2002; 225:685–692.

54. Awai K, Murao K, Ozawa A, et al. Pulmonary nodules at chest CT: effect of computer-aided diagnosis on radiologists' detection performance. Radiology. 2004; 230:347–352.

55. Chang JM, Lee HJ, Goo JM, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006; 7:57–69.

56. Zwirewich CV, Vedal S, Miller RR, Muller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991; 179:469–476.

57. Awai K, Murao K, Ozawa A, et al. Pulmonary nodules: estimation of malignancy at thin-section helical CT–effect of computer-aided diagnosis on performance of radiologists. Radiology. 2006; 239:276–284.

58. Okada K, Comaniciu D, Krishnan A. Robust anisotropic Gaussian fitting for volumetric characterization of pulmonary nodules in multislice CT. IEEE Trans Med Imaging. 2005; 24:409–423.

59. Leader JK, Warfel TE, Fuhrman CR, et al. Pulmonary nodule detection with low-dose CT of the lung: agreement among radiologists. AJR Am J Roentgenol. 2005; 185:973–978.

60. Peloschek P, Sailer J, Weber M, Herold CJ, Prokop M, Schaefer-Prokop C. Pulmonary nodules: sensitivity of maximum intensity projection versus that of volume rendering of 3D multidetector CT data. Radiology. 2007; 243:561–569.

61. Kostis WJ, Yankelevitz DF, Reeves AP, Fluture SC, Henschke CI. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology. 2004; 231:446–452.

62. Mazzone P, Obuchowski N, Mekhail T, Meziane M, Ahmad M. Lung cancer screening: is it time for a change in policy? Cleve Clin J Med. 2007; 74:441–448.

63. Kim TJ, Lee KW, Kim HY, et al. Simple pulmonary eosinophilia evaluated by means of FDG PET: the findings of 14 cases. Korean J Radiol. 2005; 6:208–213.

64. Nie Y, Li Q, Li F, Pu Y, Appelbaum D, Doi K. Integrating PET and CT information to improve diagnostic accuracy for lung nodules: a semiautomatic computer-aided method. J Nucl Med. 2006; 47:1075–1080.
65. Jeong YJ, Yi CA, Lee KS. Solitary pulmonary nodules: detection, characterization, and guidance for further diagnostic workup and treatment. AJR Am J Roentgenol. 2007; 188:57–68.

66. Hayat MA. General methods and overviews, lung carcinoma and prostate carcinoma. Sensakovic WF, Armato SG, editors. Magnetic resonance imaging of the lung: automated segmentation methods.vol 2. Dordrecht: Springer;2008. p. 219–234.

67. Abolmaali ND, Vogl TJ. Modern diagnosis of lung nodules. Radiologe. 2004; 44:472–483.
68. Schafer JF, Vollmar J, Schick F, et al. Imaging diagnosis of solitary pulmonary nodules on an open low-field MRI system: comparison of two MR sequences with spiral CT. Rofo. 2002; 174:1107–1114.
69. Schroeder T, Ruehm SG, Debatin JF, Ladd ME, Barkhausen J, Goehde SC. Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol. 2005; 185:979–984.

70. Vogt FM, Herborn CU, Hunold P, et al. HASTE MRI versus chest radiography in the detection of pulmonary nodules: comparison with MDCT. AJR Am J Roentgenol. 2004; 183:71–78.

71. Plathow C, Meinzer HP, Kauczor HU. Visualization of pulmonary nodules with magnetic resonance imaging (MRI). Radiologe. 2006; 46:260–266.
72. Peng G, Cai Z, Gao Y. The value of CT and MRI in differentiating malignant nodule from tuberculoma. Zhonghua Jie He He Hu Xi Za Zhi. 1995; 18:218–220. 255.
73. Park KY, Kim SJ, Noh TW, et al. Diagnostic efficacy and characteristic feature of MRI in pulmonary hamartoma: comparison with CT, specimen MRI, and pathology. J Comput Assist Tomogr. 2008; 32:919–925.
74. Ohno Y, Koyama H, Takenaka D, et al. Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-[fluorine-18]-fluoro-2-deoxy-D-glucose-po-sitron emission tomography (FDG-PET)/CT: comparative study of capability for management of pulmonary nodules. J Magn Reson Imaging. 2008; 27:1284–1295.

75. Schaefer JF, Vollmar J, Wiskirchen J, et al. Differentiation between malignant and benign solitary pulmonary nodules with proton density weighted and ECG-gated magnetic resonance imaging. Eur J Med Res. 2006; 11:527–533.
Figures and Tables
Fig. 1.
The image from a 61-year-old man. Peripheral cancer of the left lung. MRI of the thoracic cavity (arrows points to the tumor). (A) T2-weighted image in a coronal plane, thickness of a cut – 6 mm; (B) T2-weighted image in a coronal plane, thickness of a cut – 4 mm; (C) T2-weighted image in transversal plane, thickness of a cut-6 mm; (D) T1-weighted image in transversal plane after the intravenous introduction of contrast substance (Omniscan).

Fig. 2.
Lung cancer morbidity and lethality in Belarus, 1998, 2002, 2007 (A: male morbidity, B: male lethality, C: female morbidity, D: female lethality, E: both genders morbidity, F: both genders lethality).
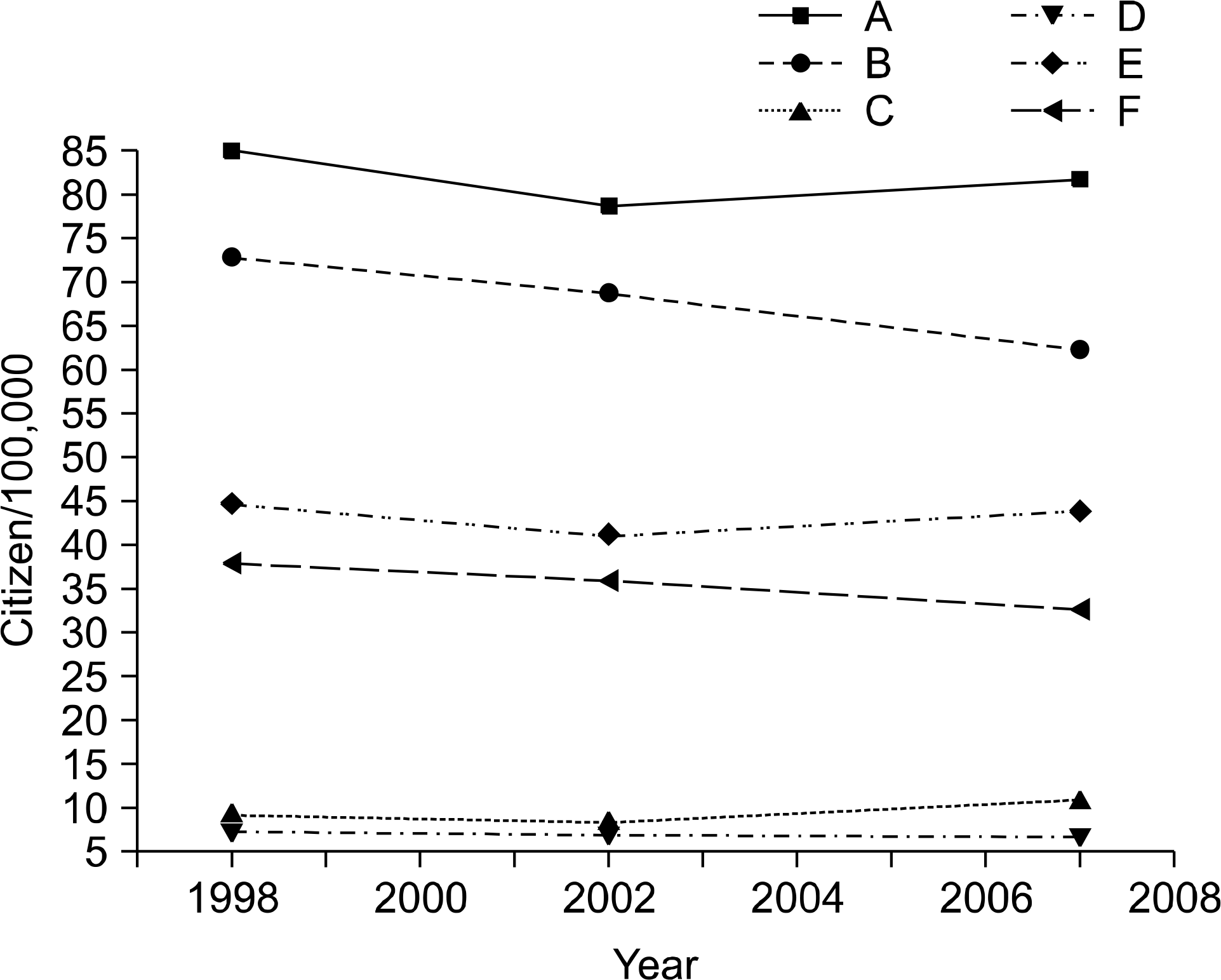
Fig. 3.
Principal causes of solitary pulmonary nodules. Hansen HH. Textbook of lung cancer. 2nd ed. London: Informa Healthcare; 2008 (46).
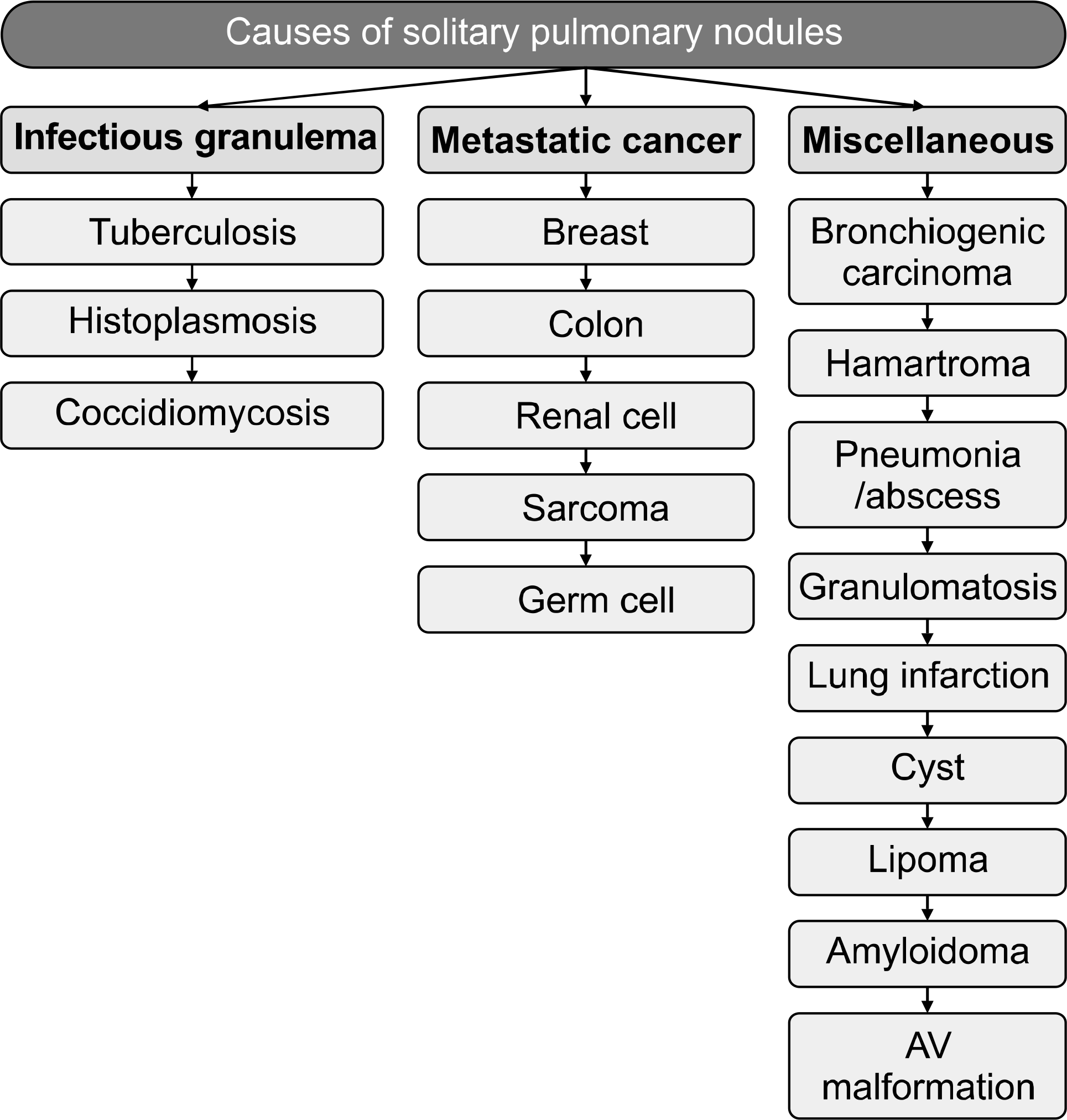
Fig. 4.
Diagram of the computerized scheme for detection of pulmonary nodules on CT images. Awai K, Murao K, Ozawa A, et al. Radiology 2006;239:276–284 (57).
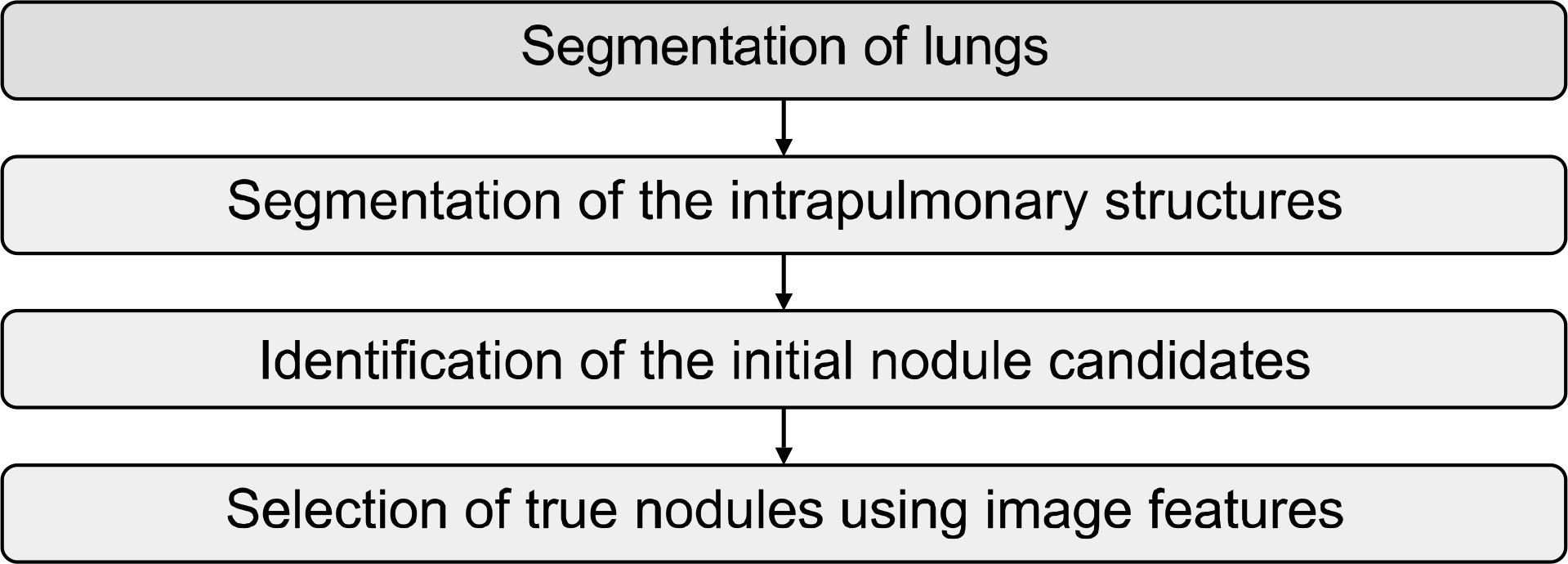
Fig. 5.
The scheme of pulmonary nodules scoring. Leader JK, Warfel TE, Fuhrman CR, et al. AJR Am J Roentgenol 2005; 185:973–978 (59).
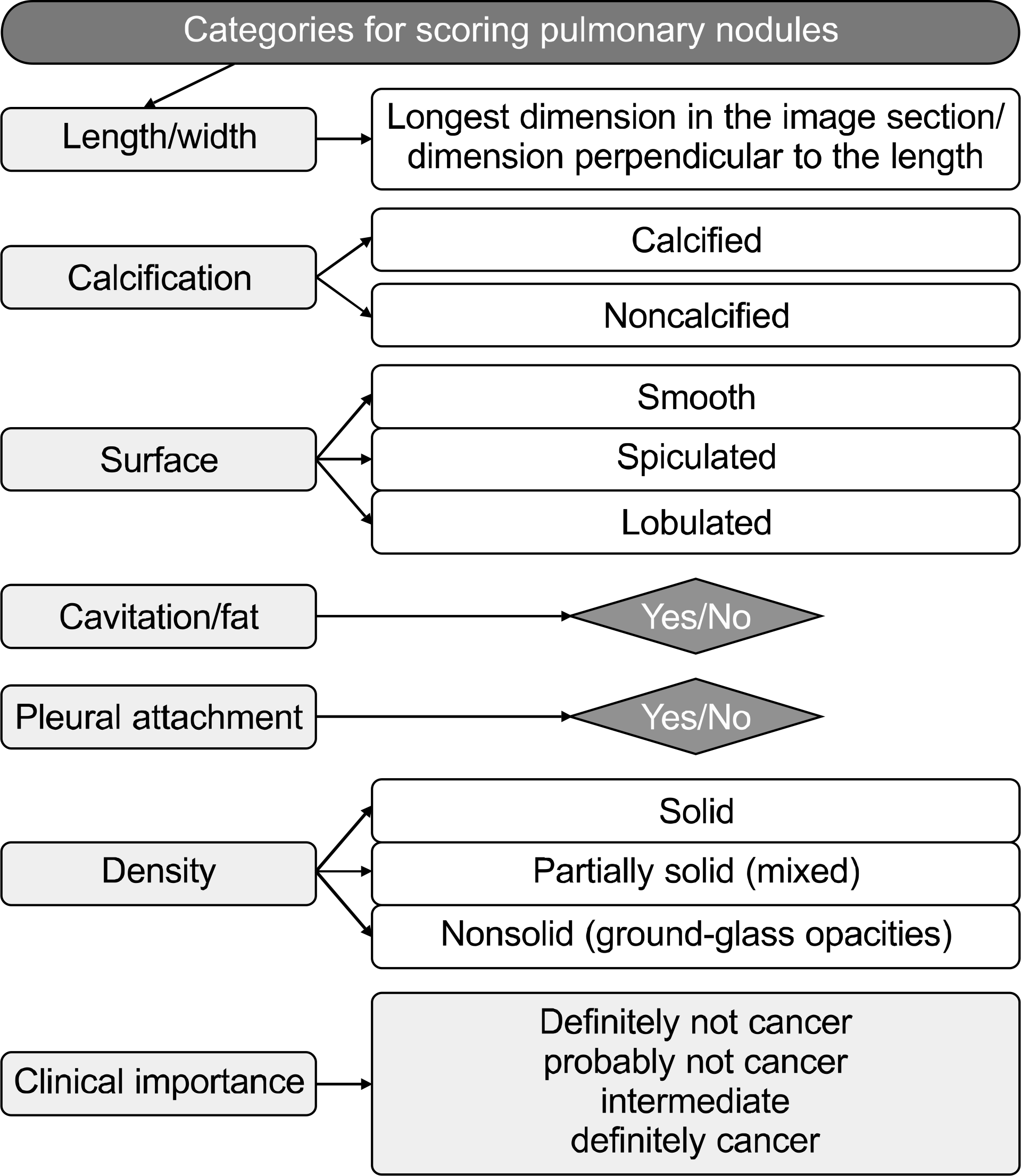
Table 1.
Volume Doubling Time of the SPN
Table 2.
Probability of Malignancy for Indeterminate Solitary Pulmonary Nodule




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download