Abstract
Purpose
Although both platinum-based drugs and third-generation drugs are commonly used as first-line therapy for patients with advanced, unresectable non-small cell lung cancer, their effectiveness and clinical outcomes vary. We investigated whether epidermal growth factor receptor (EGFR) and HER-2 were correlated with the chemoresponse and survival after treatment with a cisplatin plus paclitaxel regimen.
Materials and Methods
Forty-nine tumors were analyzed by chromogenic in situ hybridization (CISH) for EGFR and HER-2 gene amplification.
Results
Twenty-eight patients (57%) achieved a partial response (PR), 13 (27%) showed stable disease (SD) and 8 (16%) had progressive disease (PD). EGFR and HER-2 amplification was identified in 43% and 57% of the tumors, respectively. EGFR amplification revealed no association with either a chemoresponse or survival, whereas HER-2 was amplified more frequently in the patients with PD (88% vs. 54%, respectively, p=0.06) and in the patients with shorter survival (12 months vs. 20 months respectively, p=0.027).
Go to : 
References
1. Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001; 93:277–283.

2. Brundage MD, Davies D, Mackillop WJ. Prognostic factors in nonsmall cell lung cancer: a decade of progress. Chest. 2002; 122:1037–1057.
3. Suzuki S, Dobashi Y, Sakurai H, Nishikawa K, Hanawa M, Ooi A. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: an immunohistochemical and fluorescence in situ hybridization study. Cancer. 2005; 103:1265–1273.
4. Toschi L, Cappuzzo F. Understanding the new genetics of responsiveness to epidermal growth factor receptor tyrosine kinase inhibitors. Oncologist. 2007; 12:211–220.

5. Hirsch FR, Varella-Garcia M, Cappuzzo F, et al. Combination of EGFR gene copy number and protein expression predicts outcome for advanced nonsmall-cell lung cancer patients treated with gefitinib. Ann Oncol. 2007; 18:752–760.

6. Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptors shares chromosomal location with neu oncogene. Science. 1985; 230:1132–1139.
7. Mé nard S, Valagussa P, Pilotti S, et al. Response to cyclo-hosphamide, methotrexate, and fluorouracil in lymph nodepositive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol. 2001; 19:329–335.
8. Meden H, Marx D, Roegglen T, Schauer A, Kuhn W. Overexpression of the oncogene c-erbB-2 (HER2/neu) and response to chemotherapy in patients with ovarian cancer. Int J Gynecol Pathol. 1998; 17:61–65.

9. Tsai CM, Levitzki A, Wu LH, et al. Enhancement of chemosensitivity by tyrphostin AG825 in high-p185 (neu) expressing nonsmall cell lung cancer cells. Cancer Res. 1996; 56:1086–1074.
10. Greatens TM, Niehans GA, Rubins JB, et al. Do molecular markers predict survival in nonsmall-cell lung cancer? Am J Respir Crit Care Med. 1998; 157:1093–1097.

11. Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edition (1997). Union internationale contre le cancer and the American joint committee on cancer. Cancer. 1997; 80:1803–1804.
12. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.
13. Tanner M, Gancberg D, Di Leo A, et al. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000; 157:1467–1472.
14. Baselga J, Seidman AD, Rosen PP, Norton L. HER2 overexpression and paclitaxel sensitivity in breast cancer: therapeutic implications. Oncology (Williston Park). 1997; 11:43–48.
15. Schneider S, Uchida K, Brabender J, et al. Downregulation of TS, DPD, ERCC1, GST-Pi, EGFR, and HER2 gene expression after neoadjuvant three-modality treatment in patients with esophageal cancer. J Am Coll Surg. 2005; 200:336–344.

16. Jä nne PA, Gurubhagavatula S, Yeap BY, et al. Outcomes of patients with advanced nonsmall cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer. 2004; 44:221–230.

17. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness if nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.
18. Cappuzzo F. Should every lung cancer patients be tested for EGFR mutation? Expert Opin Ther Targets. 2006; 10:789–791.
19. Tsutsui S, Kataoka A, Ohno S, Murakami S, Kinoshita J, Hachitanda Y. Prognostic and predictive value of epidermal growth factor receptor in recurrent breast cancer. Clin Cancer Res. 2002; 8:3454–3460.
20. Hancock MC, Langton BC, Chan T, et al. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diaminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991; 51:4575–4580.
21. Tsai CM, Yu D, Chang KT, et al. Enhanced chemoresistance by elevation of p185neu levels in HER-2/neu-transfected human lung cancer cells. J Natl Cancer Inst. 1995; 87:682–684.

22. Selvaggi G, Scagliotti GV, Torri V, et al. HER-2/neu overexpression in patients with radically resected nonsmall cell lung carcinoma: impact on longterm survival. Cancer. 2002; 94:2669–2674.
23. Harpole DH Jr, Marks JR, Richards WG, Herndon JE 2nd, Sugarbaker DJ. Localized adenocarcinoma of the lung: oncogene expression of erbB-2 and p53 in 150 patients. Clin Cancer Res. 1995; 1:659–664.
24. Pfeiffer P, Clausen PP, Andersen K, Rose C. Lack of prognostic significance of epidermal growth factor receptor and the oncoprotein p185HER–2 in patients with systemically untreated nonsmall-cell lung cancer: an immunohistochemical study on cryosections. Br J Cancer. 1996; 74:86–91.

Go to : 
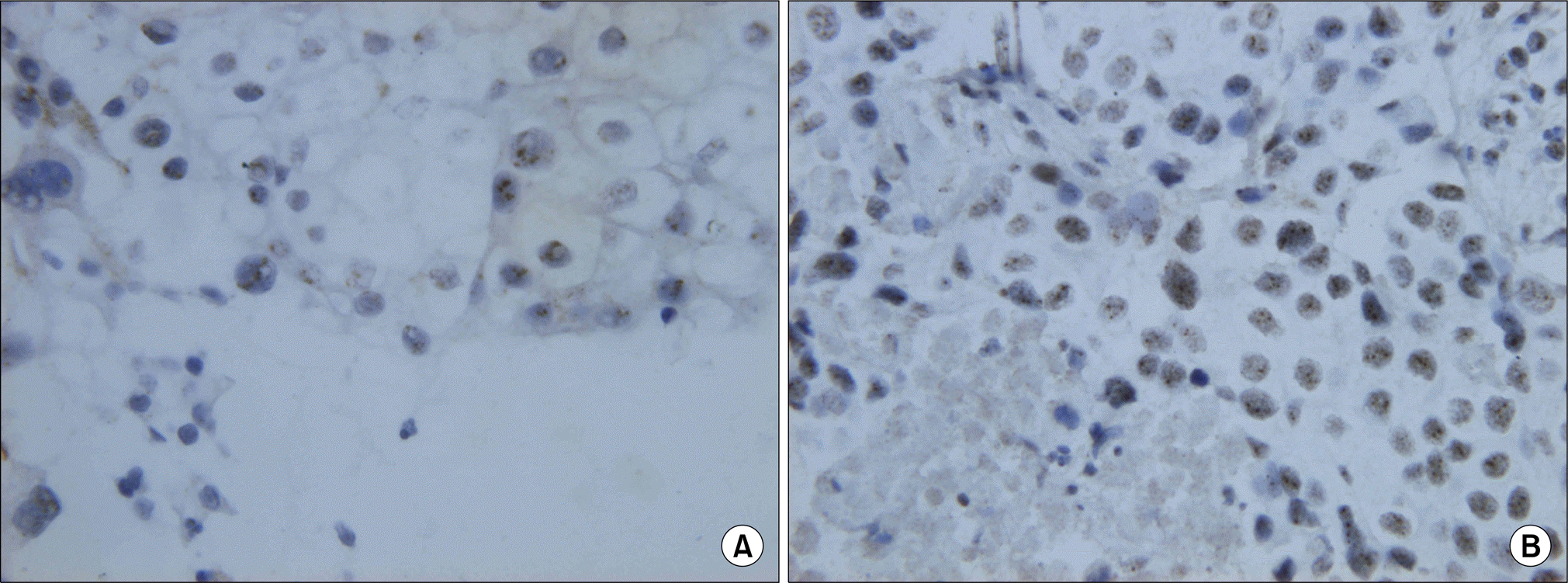 | Fig. 1.EGFR (A) and HER-2 (B) gene amplification by chromogenic in situ hybridization. A typical gene amplification appears as a cluster of multiple individual gene copies (CISH, ×400). EGFR: epidermal growth factor receptor. |
Tables
Table 1.
Clinicopathologic Features and Their Relationship with Tumor Response to Chemotherapy
Table 2.
Association between Clinicopathologic Variables and Gene Amplification of EGFR and HER-2
Table 3.
Association between Clinicopatholgic Variables and Median Survival
Table 4.
Univariate and Multivariate Analyses of Cox Proportional Hazard Model




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download