Abstract
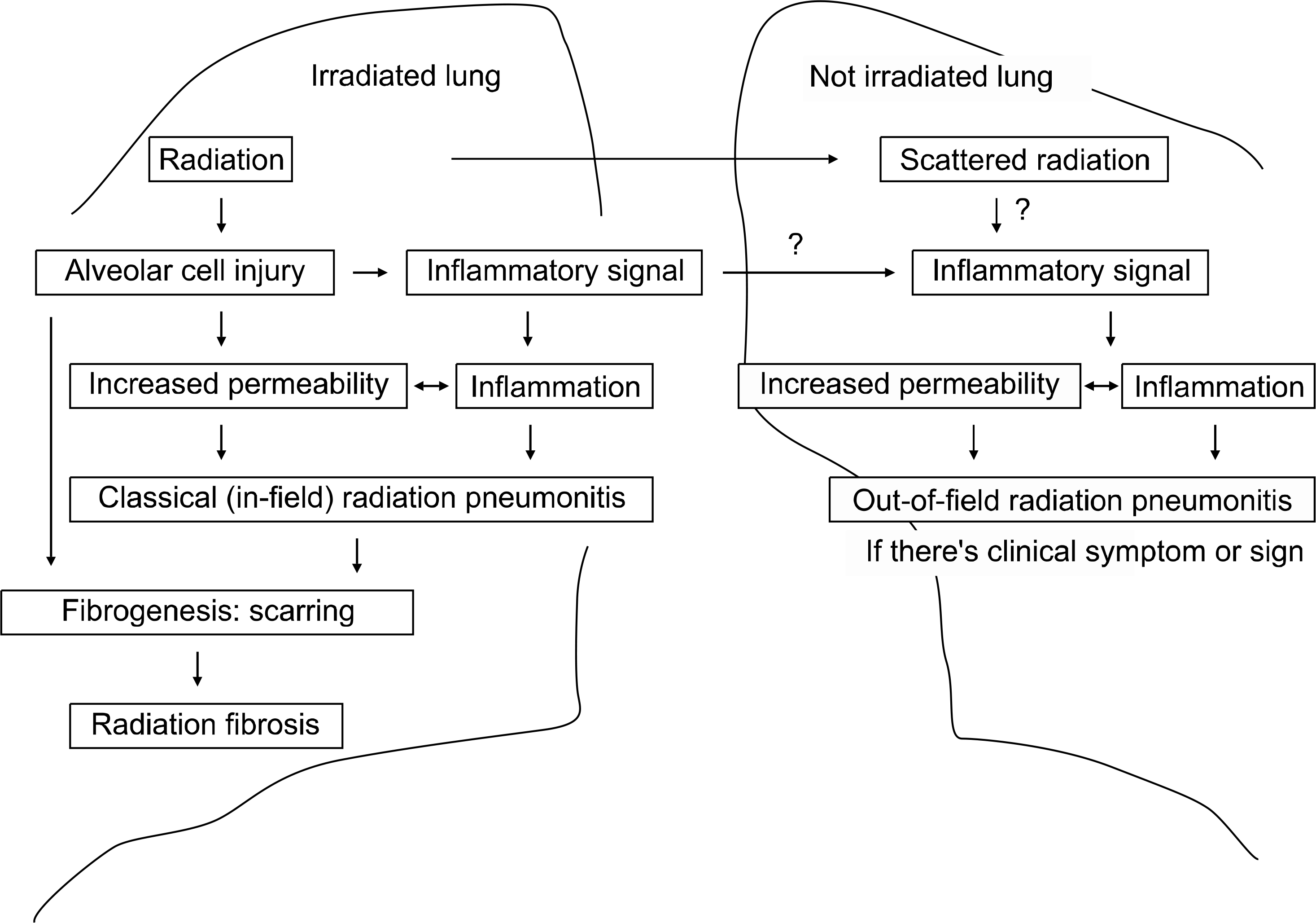
Radiation therapy is one of most important therapeutic modalities for thoracic malignancies. However, radiation-induced lung damage, such as radiation pneumonitis or fibrosis, is a main dose-limiting factor when irradiating the thorax. The radiation over threshold dose results in damage to pneumocytes and endothelial cells and the inflammatory changes following the damage lead to necrosis of damaged tissue, which are then replaced by fibrotic tissue. There is diffuse lung damage and edema on histopathologic inspection; however, the tissue damage and edema is not specific for radiation injury and we are far from a reliable pathogenic model. Many parameters have been evaluated for predicting radiation pneumonitis and the most consistent predictor is cumulative radiation dose to normal lung tissue. The combination of chemotherapy probably increases the incidence and severity of radiation pneumonitis; however, this is not clear. Efforts to reduce the radiation dose to normal lung tissue using new radiotherapy techniques can reduce the incidence and severity of radiation-induced lung damage. Many biological agents have been tried to prevent and treat radiation pneumonitis; however, more data is needed.
References
1. Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1991; 21:355–360.

2. Marks LB, Munley MT, Bentel GC, et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys. 1997; 39:563–570.

3. Morgan GW, Freeman AP, McLean RG, Jarvie BH, Giles RW. Late cardiac, thyroid, and pulmonary sequelae of mantle radiotherapy for Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1985; 11:1925–1931.

4. Penn CR, Hope-Stone HF. The role of radiotherapy in the management of malignant thymoma. Br J Surg. 1972; 59:533–539.

5. Postoperative radiotherapy in nonsmall-cell lung cancer: systematic review and metaanalysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998; 352:257–263.
6. Mehta V. Radiation pneumonitis and pulmonary fibrosis in nonsmall-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005; 63:5–24.

7. Mah K, Van Dyk J, Keane T, Poon PY. Acute radiation-induced pulmonary damage: a clinical study on the response to fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1987; 13:179–188.

8. Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995; 31:361–369.

9. Martin C, Romero S, Sanchez-Paya J, Massuti B, Arriero JM, Hernandez L. Bilateral lymphocytic alveolitis: a common reaction after unilateral thoracic irradiation. Eur Respir J. 1999; 13:727–732.

10. Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006; 66:1281–1293.

11. McDonald S, Rubin P, Phillips TL, Marks LB. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys. 1995; 31:1187–1203.

12. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31:1341–1346.

13. Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000; 47:13–47.

14. Fan M, Marks LB, Hollis D, et al. Can we predict radiation-induced changes in pulmonary function based on the sum of predicted regional dysfunction? J Clin Oncol. 2001; 19:543–550.

15. Fan M, Marks LB, Lind P, et al. Relating radiation-induced regional lung injury to changes in pulmonary function tests. Int J Radiat Oncol Biol Phys. 2001; 51:311–317.

16. Miller KL, Kocak Z, Kahn D, et al. Preliminary report of the 6-minute walk test as a predictor of radiation-induced pulmonary toxicity. Int J Radiat Oncol Biol Phys. 2005; 62:1009–1013.

17. Reckzeh B, Merte H, Pfluger KH, Pfab R, Wolf M, Havemann K. Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for nonsmall-cell lung cancer. J Clin Oncol. 1996; 14:1071–1076.

18. Yu TK, Whitman GJ, Thames HD, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst. 2004; 96:1676–1681.

19. Segawa Y, Takigawa N, Kataoka M, Takata I, Fujimoto N, Ueoka H. Risk factors for development of radiation pneumonitis following radiation therapy with or without chemotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 1997; 39:91–98.

20. Tsujino K, Hirota S, Kotani Y, et al. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: Dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006; 64:1100–1105.

21. Roach M 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995; 13:2606–2612.

22. Lind PA, Marks LB, Hollis D, et al. Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol Phys. 2002; 54:340–347.

23. Seppenwoolde Y, De Jaeger K, Boersma LJ, Belderbos JS, Lebesque JV. Regional differences in lung radiosensitivity after radiotherapy for nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 60:748–758.

24. Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the longterm prognosis. Int J Radiat Oncol Biol Phys. 2001; 49:649–655.

25. Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with nonsmall-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002; 54:735–741.

26. Brooks BJ Jr, Seifter EJ, Walsh TE, et al. Pulmonary toxicity with combined modality therapy for limited stage small-cell lung cancer. J Clin Oncol. 1986; 4:200–209.
27. Lee JS, Scott C, Komaki R, et al. Concurrent chemoradiation therapy with oral etoposide and cisplatin for locally advanced inoperable nonsmall-cell lung cancer: radiation therapy oncology group protocol 91–06. J Clin Oncol. 1996; 14:1055–1064.

28. Byhardt RW, Scott C, Sause WT, et al. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1998; 42:469–478.

29. Yamada M, Kudoh S, Hirata K, Nakajima T, Yoshikawa J. Risk factors of pneumonitis following chemoradiotherapy for lung cancer. Eur J Cancer. 1998; 34:71–75.

30. Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable nonsmall-cell lung cancer. N Engl J Med. 1992; 326:524–530.

31. Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III nonsmall-cell lung cancer: a randomized study. J Clin Oncol. 1996; 14:1065–1070.

32. Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000; 48:89–94.

33. Rubin P, Siemann DW, Shapiro DL, Finkelstein JN, Penney DP. Surfactant release as an early measure of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1983; 9:1669–1673.

34. Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995; 33:99–109.

35. Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002; 12:26–33.

36. Ibuki Y, Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med. 1997; 22:1029–1035.
37. Hong JH, Jung SM, Tsao TC, et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol. 2003; 79:159–167.

38. Gross NJ. Radiation pneumonitis in mice. Some effects of corticosteroids on mortality and pulmonary physiology. J Clin Invest. 1980; 66:504–510.

39. Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005; 201:925–935.

40. Boerma M, van der Wees CG, Vrieling H, et al. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics. 2005; 6:6.

41. Boerma M, Wang J, Wondergem J, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res. 2005; 65:3100–3107.

42. Christiansen H, Batusic D, Saile B, et al. Identification of genes responsive to gamma radiation in rat hepatocytes and rat liver by cDNA array gene expression analysis. Radiat Res. 2006; 165:318–325.

43. Christiansen H, Sheikh N, Saile B, et al. x-Irradiation in rat liver: consequent upregulation of hepcidin and downregulation of hemojuvelin and ferroportin-1 gene expression. Radiology. 2007; 242:189–197.

44. Anscher MS, Peters WP, Reisenbichler H, Petros WP, Jirtle RL. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993; 328:1592–1598.
45. Anscher MS, Marks LB, Shafman TD, et al. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. J Clin Oncol. 2001; 19:3758–3765.

46. De Jaeger K, Seppenwoolde Y, Kampinga HH, Boersma LJ, Belderbos JS, Lebesque JV. Significance of plasma transforming growth factor-beta levels in radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 58:1378–1387.
47. Anscher MS, Kong FM, Andrews K, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998; 41:1029–1035.
48. Anscher MS, Kong FM, Marks LB, Bentel GC, Jirtle RL. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997; 37:253–258.

49. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000; 342:1350–1358.
50. Burger A, Loffler H, Bamberg M, Rodemann HP. Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol. 1998; 73:401–408.
51. Vujaskovic Z, Groen HJ. TGF-beta, radiation-induced pulmonary injury and lung cancer. Int J Radiat Biol. 2000; 76:511–516.
52. Fine A, Goldstein RH. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987; 262:3897–3902.

53. Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994; 28:621–631.
54. Rube CE, Uthe D, Schmid KW, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000; 47:1033–1042.
55. Girinsky T. Effects of ionizing radiation on the blood vessel wall. J Mal Vasc. 2000; 25:321–324.
56. Gridley DS, Bonnet RB, Bush DA, et al. Time course of serum cytokines in patients receiving proton or combined photon/proton beam radiation for resectable but medically inoperable nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 60:759–766.

57. Vujaskovic Z, Anscher MS, Feng QF, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001; 50:851–855.

58. Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996; 145:762–767.
59. Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Res. 1997; 57:2096–2099.
60. Nozaki Y, Hasegawa Y, Takeuchi A, et al. Nitric oxide as an inflammatory mediator of radiation pneumonitis in rats. Am J Physiol. 1997; 272:L651–658.

61. Giaid A, Lehnert SM, Chehayeb B, Chehayeb D, Kaplan I, Shenouda G. Inducible nitric oxide synthase and nitrotyrosine in mice with radiation-induced lung damage. Am J Clin Oncol. 2003; 26:e67–72.

62. Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for nonsmall cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999; 45:323–329.

63. Jenkins P, D'Amico K, Benstead K, Elyan S. Radiation pneumonitis following treatment of nonsmall-cell lung cancer with continuous hyperfractionated accelerated radiotherapy (CHART). Int J Radiat Oncol Biol Phys. 2003; 56:360–366.

64. Tsujino K, Hirota S, Endo M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003; 55:110–115.

65. Claude L, Perol D, Ginestet C, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in nonsmall-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004; 71:175–181.

66. Kim TH, Cho KH, Pyo HR, et al. Dosevolumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005; 235:208–215.

67. Lee SW, Choi EK, Lee JS, et al. Phase II study of three-dimensional conformal radiotherapy and concurrent mitomycin-C, vinblastine, and cisplatin chemotherapy for Stage III locally advanced, unresectable, nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003; 56:996–1004.

68. Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989; 16:1623–1630.
69. Lyman JT, Wolbarst AB. Optimization of radiation therapy, III: A method of assessing complication probabilities from dose-volume histograms. Int J Radiat Oncol Biol Phys. 1987; 13:103–109.

70. Martel MK, Ten Haken RK, Hazuka MB, Turrisi AT, Fraass BA, Lichter AS. Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis. Int J Radiat Oncol Biol Phys. 1994; 28:575–581.

71. Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001; 50:899–908.
72. Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-beta1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999; 86:1712–1719.
73. Kong FM, Washington MK, Jirtle RL, Anscher MS. Plasma transforming growth factor-beta 1 reflects disease status in patients with lung cancer after radiotherapy: a possible tumor marker. Lung Cancer. 1996; 16:47–59.
74. Chen Y, Rubin P, Williams J, Hernady E, Smudzin T, Oku-nieff P. Circulating IL-6 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2001; 49:641–648.

75. Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in nonsmall-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet. 1997; 350:161–165.
76. Sunyach MP, Falchero L, Pommier P, et al. Prospective evaluation of early lung toxicity following three-dimensional conformal radiation therapy in nonsmall-cell lung cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2000; 48:459–463.

77. Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 58:1258–1267.

78. Scrimger RA, Tome WA, Olivera GH, Reckwerdt PJ, Mehta MP, Fowler JF. Reduction in radiation dose to lung and other normal tissues using helical tomotherapy to treat lung cancer, in comparison to conventional field arrangements. Am J Clin Oncol. 2003; 26:70–78.

79. Bonnet RB, Bush D, Cheek GA, et al. Effects of proton and combined proton/photon beam radiation on pulmonary function in patients with resectable but medically inoperable nonsmall cell lung cancer. Chest. 2001; 120:1803–1810.

80. Kadono K, Homma T, Kamahara K, et al. Effect of heavy-ion radiotherapy on pulmonary function in stage I nonsmall cell lung cancer patients. Chest. 2002; 122:1925–1932.

81. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006; 24:4833–4839.

82. Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002; 13:181–209.

83. Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable nonsmall-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004; 58:1369–1377.

84. Antonadou D, Petridis A, Synodinou M, et al. Amifostine reduces radiochemotherapy-induced toxicities in patients with locally advanced nonsmall cell lung cancer. Semin Oncol. 2003; 30:2–9.

85. Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced nonsmall-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98–01. J Clin Oncol. 2005; 23:2145–2154.

86. Wang LW, Fu XL, Clough R, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res. 2000; 153:405–410.

87. Anscher MS, Thrasher B, Zgonjanin L, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008; 71:829–837.
88. Ozturk B, Egehan I, Atavci S, Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2004; 58:213–219.

89. Hallahan DE, Geng L, Shyr Y. Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst. 2002; 94:733–741.

90. Kang SK, Rabbani ZN, Folz RJ, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003; 57:1056–1066.

91. Vijayalaxmi. Reiter RJ, Tan DX, Herman TS, Thomas CR Jr. Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004; 59:639–653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download