Abstract
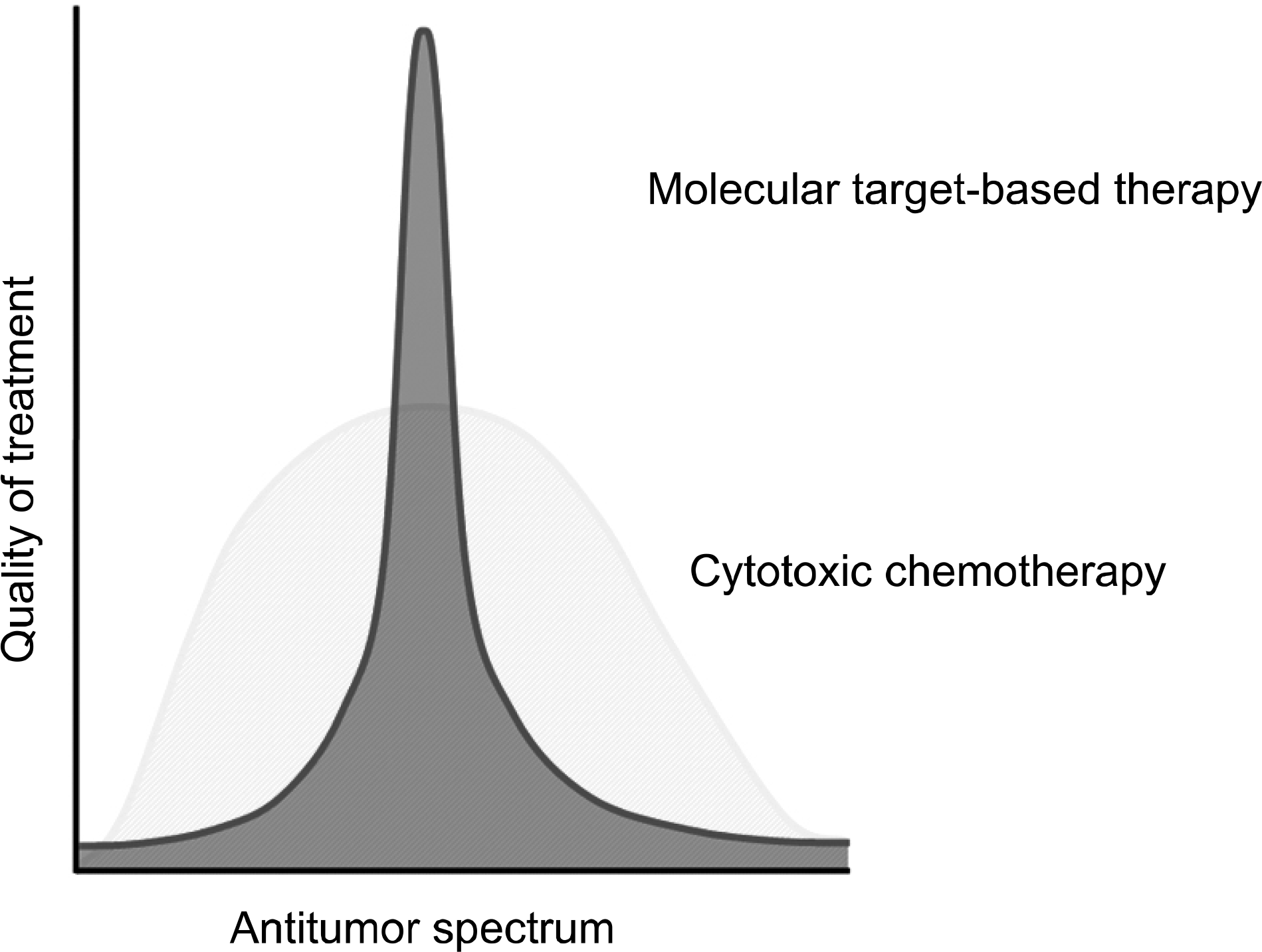
Many clinical trials of molecular target drugs have been done against advanced lung cancer, however, majority did not meet the primary endpoint. Positive studies of EGFR-TKI such as BR21 and Interest used unselected populations of non-small cell lung cancer. It was quite difficult to explain why they were positive. In the present review, the difficulties of clinical trial design in molecular target drugs were discussed based on the differences of the magnitude of antitumor activity and the target tumor cell population between cytotoxic drugs and molecular target therapy.
Go to : 
References
1. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced nonsmall-cell lung cancer. N Engl J Med. 2002; 346:92–98.

2. Kelly K, Crowley J, Bunn PA Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced nonsmall-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001; 19:3210–3218.

3. Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced nonsmall-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007; 18:317–323.

5. Shepherd FA, Giaccone G, Seymour L, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol. 2002; 20:4434–4439.

6. Adjei AA, Mauer A, Bruzek L, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced nonsmall-cell lung cancer. J Clin Oncol. 2003; 21:1760–1766.

7. Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive nonsmall-cell lung cancer. Ann Oncol. 2004; 15:19–27.

8. Williamson SK, Crowley JJ, Lara PN Jr, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced nonsmall-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005; 23:9097–9104.

9. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.

10. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500.
11. Arao T, Fukumoto H, Takeda M, et al. Small in-frame deletion in the epidermal growth factor receptor as a target for ZD6474. Cancer Res. 2004; 64:9101–9104.

12. Johnson BE, Janne PA. Selecting patients for epidermal growth factor receptor inhibitor treatment: A FISH story or a tale of mutations? J Clin Oncol. 2005; 23:6813–6816.

13. Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002; 20:2240–2250.

14. Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in nonsmall-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002; 20:3815–3825.

15. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced nonsmall-cell lung cancer (The IDEAL 1 trial). J Clin Oncol. 2003; 21:2237–2246.
16. Giaccone G, Rodriguez JA. EGFR inhibitors: what have we learned from the treatment of lung cancer? Nature Clin Practive Oncol. 2005; 2:554–561.

17. Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced nonsmall-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol. 2004; 22:777–784.
18. Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced nonsmall-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004; 22:785–794.
19. Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced nonsmall-cell lung cancer. J Clin Oncol. 2005; 23:5892–5899.

20. Gatzemeier U, Pluzanska A, Szczesna A, et al. Results of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced nonsmall-cell lung cancer (NSCLC). J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). 2004; 22(14S):7010.
21. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005; 353:123–132.

22. Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced nonsmall-cell lung cancer: results from a randomized, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 336:1527–1537.
23. Chang A, Parikh P, Thongprasert S, et al. Gefitinib (IRESSA) is patients of Asian origin with refractory advanced nonsmall cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol. 2006; 1:847–855.
24. Kelly K, Gaspar LE, Chansky K, et al. Low incidence of pneumonitis on SWOG 0023: A preliminary analysis of an ongoing phase III trial of concurrent chemoradiotherapy followed by consolidation docetaxel and Iressa/placebo maintenance in patients with inoperable stage III nonsmall cell lung cancer. J Clin Oncol, 2005 ASCO Annual Meeting Proceedings. 2005; 23(16S):7058.
25. Tsuboi M, Kato H, Nagai K, et al. Gefitinib in the adjuvant setting: safety results from a phase III study in patients with completely resected nonsmall cell lung cancer. Anticancer Drugs. 2005; 16:1123–1128.

26. Niho S, Ichinose Y, Tamura T, et al. Results of a randomized Phase III study to compare the overall survival of gefitinib (IRESSA) versus docetaxel in Japanese patients with non-small-cell lung cancer who failed one or two chemotherapy regimens. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):LBA7509.
27. Douillard JY, Kim E, Hirsh V, et al. Gefitinib (Iressa) versus docetaxel in patients with locally advanced or metastatic NSCLC pretreated with platinum-based chemotherapy: a randomized, open-label phase III study (Interest). J Thorac Oncol. 2007; 2:S305–S306.
28. Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004; 64:8919–8923.
29. Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in nonsmall-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005; 23:2493–2501.

30. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–346.

31. Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with nonsmall-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005; 23:2513–2520.

32. Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent nonsmall-cell lung cancer. J Clin Oncol. 2005; 23:6829–6837.

33. Giaccone G, Janmeat M, Ruiz MG, et al. EGFR mutations do not accurately predict response to erlotinib in first line monotherapy treatment of advanced non small cell lung cancer. Lung Cancer. 2005; 49:S244. (P-485).
34. Sequist LV, Joshi VA, Janne PA, et al. Response to treatment and survival of patients with nonsmall cell lung cancer undergoing somatic EGFR mutation testing. Oncologist. 2007; 12:90–98.

35. Kaye FJ. A curious link between epidermal growth factor receptor amplification and survival: effect of "allele dilution" on gefitinib sensitivity? J Natl Cancer Inst. 2005; 97:621–623.

36. Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in nonsmall-cell lung caner: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005; 23:857–865.
37. Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786–792.
38. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PloS Med. 2005; 2:e73.

39. Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in nonsmall-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005; 31:8081–8092.

40. Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in nonsmall-cell lung cancer. J Natl Cancer Inst. 2005; 97:643–655.

41. Hirsch FR, Varella-Garcia M, Bunn PA Jr, et al. Epidermal growth factor receptor in nonsmall-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003; 21:3798–3807.

42. Cappuzzo F, Varella-Garcia M, Shigematsu H, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive nonsmall-cell lung cancer patient. J Clin Oncol. 2005; 23:5007–5018.
43. Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005; 23:6838–6845.

44. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005; 353:133–144.

45. Rosell R, Daniel C, Ramlau R, et al. Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced nonsmall-cell lung cancer (NSCLC). J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). 2004; 22(14S):7012.

46. Herbst RS, Chansky K, Kelly K, et al. A phase II randomized selection trial evaluating concurrent chemotherapy plus cetuximab or chemotherapy followed by cetuximab in patients with advanced NSCLC: Final results of SWOG 0342. Proc. ASCO. 25(395s):7545. 2007.
47. Blumenschein G, Monghan J, Curran W, et al. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (pts) with stage III A/B nonsmall cell lung cancer (NSCLC): An interim report of the RTOG 0324 trial. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):7531.

48. Butts CA, Bodkin D, Middleman EL, et al. Gemcitabine/platinum alone or in combination with cetuximab as a first-line treatment for advanced nonsmall cell lung cancer (NSCLC). J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):7539.
50. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–2342.

51. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007; 25:1539–1544.

52. Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic nonsmall-cell lung cancer. J Clin Oncol. 2004; 22:2184–2191.

53. Sandler AB, Gray R, Brahmer J, et al. Randomized phase II/III trial of paclitaxel plus carboplatin with or without bevacizumab in patients with advanced non-squamous NSCLC: An Eastern Cooperative Oncology Group Trial-E4599. Proc. ASCO. 23(2s(LBA4)):2005.
54. Manegold C, von Pawel J, Zatlaukal P, et al. Randomized double-blind multicenter phase III study of bevacizumab in combination with cisplatin and gemcitabine in chemotherapy naive patients with advanced or recurrent non-squamous nonsmall cell lung cancer (NSCLC): BO17704. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):LBA7514.
55. Adjei AA, Molina JR, Hillman SL, et al. A front-line window of opportunity phase II study of sorafenib in patients with advanced nonsmall cell lung cancer: A North Central Cancer Treatment Group. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):7547.
56. Brahmer JR, Govindan R, Novells S, et al. Efficacy and safety of continuous daily sunitinib dosing in previously treated advanced nonsmall cell lung cancer (NSCLC): Results from a phase II study. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):7542.

57. Arnold AM, Smylie M, Ding K, et al. Randomized phase II study of maintenance vandetanib (ZD6474) in small cell lung cancer (SCLC) patients who have a complete or partial response to induction therapy: NCIC GTG BR.20. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007; 25(18S):7522.
Go to : 
Figures and Tables
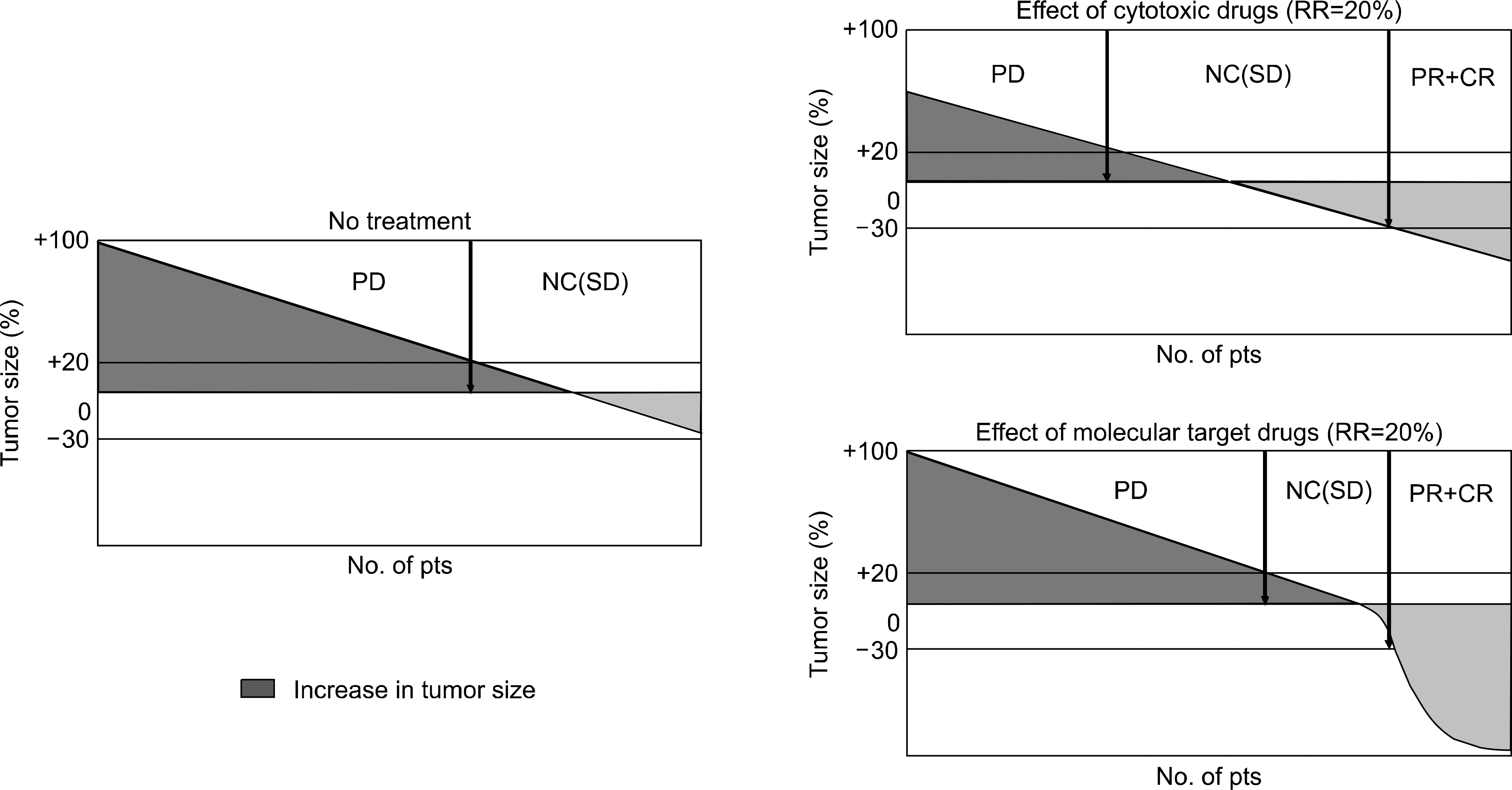 | Fig. 2.Difference in the effect of cytotoxic drugs and molecular target drugs (waterfall plots). |
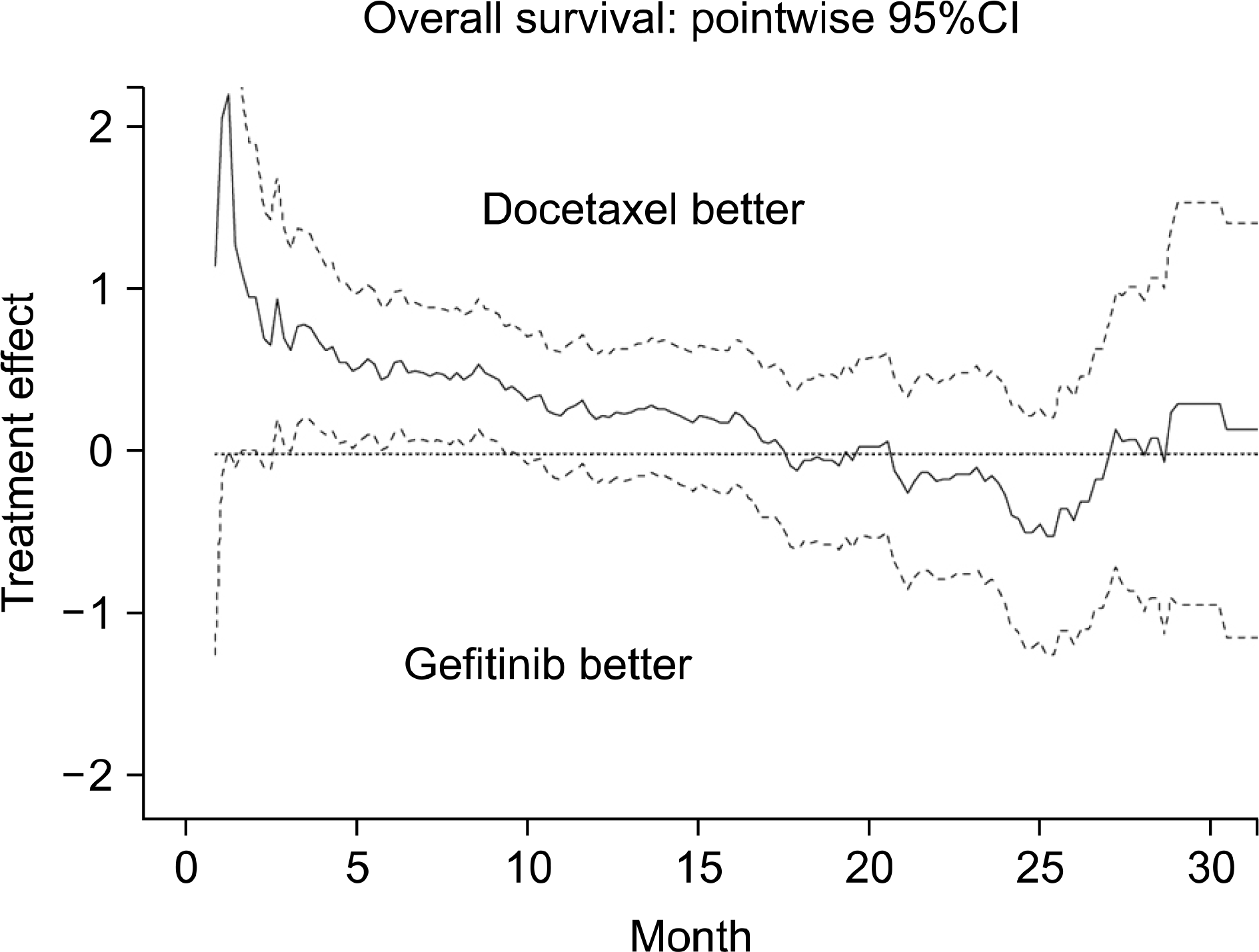
 | Fig. 4.Treatment effect at each time point. Analysed by Prof. Masahiro Takeuchi, Kitasato University, Division of Biostatistics & Division of Pharmaceutical Medicine. Courtesy of Prof. M. Takeuchi, Kitasato University. |
Table 1.
Molecular Target-based Therapy in Lung Cancer
Table 2.
RCTs (Randomized Clinical Trials) of Erlotinib & Gefitinib




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download