초록
Purpose
To evaluate the compliance of patients who underwent complete resection of non-small cell lung cancer (NSCLC) with adjuvant chemotherapy.
Materials and Methods
Between January 2004 and May 2006, patients who underwent a complete resection for NSCLC were referred to oncologists for adjuvant chemotherapy. Three or 4 cycles of platinum-based adjuvant chemotherapy was then performed according to the protocol or the preference of the oncologists.
Results
Two hundred and thirty-two patients were enrolled in this study. The median age of the study group was 60.9 years and 76.7 % of the patients enrolled were male. 34.9%, 28.8% and 36.2% of the patients were in stage IB, II and III respectively. In addition, 142 of the patients (61.2%) completed all planned cycles, whereas 65 patients (28%) received no therapy. The causes of start failure for adjuvant chemotherapy consisted of decreased postoperative performance status (n=39), refusal (n=13) and distant metastasis at the initial follow-up (n=2). The causes of cessation during adjuvant chemotherapy included the occurrence of severe adverse effects (n=12), aggravation of the disease with newly developed metastasis (n=4) and others (n=6). The mortality related to the adjuvant chemotherapy was 1.3 % (n=3), all of the fatalities were due to pneumonia and sepsis. Univariate analysis showed that age, postoperative complications and pathologic staging were the significant factors that determined whether the adjuvant chemotherapy was completed. Multivariate analysis demonstrated statistically significant differences in compliance when age and pathologic staging were considered.
Conclusion
Adjuvant chemotherapy for completely resected NSCLC was performed with satisfactory compliance in approximately 60% of the patients included in this study, and age plays an important role in the compliance of adjuvant chemotherapy. Elderly subsets will be examined to help determine the effect of age on compliance and outcome. In addition, the medical oncologist tended to complete the adjuvant chemotherapy for more advanced cases of lung cancer than for stage IB lung cancer.
Go to : 
References
1. Non-small Cell Lung Cancer Collaborative Group. chemotherapy in nonsmall cell lung cancer: a metaanalysis using updated data on individual patients from 52 randomised clinical trials. Br Med J. 1995; 311:899–909.
2. Keller S, Adak S, Wagner H, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non small cell lung cancer. N Engl J Med. 2000; 343:1217–1222.
3. Waller D, Fairlamb DJ, Gower N, et al. The Big Lung Trial (BLT): determining the value of cisplatin-based chemotherapy for all patients with non small cell lung cancer (NSCLC). Preliminary results in the surgical setting. Proc ASCO. 2003; 22:A2543.
4. Scagliotti GV, Fossati R, Torri V, et al. Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer-Lung Cancer Cooperative Group Investigators. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA nonsmall-cell lung cancer. J Natl Cancer Inst. 2003; 95:1453–1461.
5. Arriagada R, Bergman B, Dunant A, et al. The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected nonsmall cell lung cancer. N Engl J Med. 2004; 350:351–360.
6. Winton TL, Livingston R, Johnson D, et al. A prospective randomized trial of adjuvant vinorelbine (VIN) plus cisplatin (CIS) in completely resected stage IB and II non small cell lung cancer (NSCLC) Intergroup JBR.10. N Engl J Med. 2005; 352:2589–2597.
7. Strauss GM, Herndon J, Maddaus MA, et al. Randomized clinical trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in Stage IB nonsmall cell lung cancer: report of Cancer and Leukemia Group B(CALBG) Protocol 9633. J Clin Oncol. 2004; 22:621S.
8. Douillard JY, Rosell R, Delena M, Legroumellec A, Torres A, Carpagnano F, et al. ANITA: phase III adjuvant vinorelbine (N) and cisplatin (P) versus observation (OBS) in completely resected (stage I-III) nonsmall-cell lung cancer (NSCLC) patients (pts): final results after 70 month median follow-up. On behalf of the Adjuvant Navelbine International Trialist Association. J Clin Concol. 2005; 23:165.
9. Holmes EC. The Lung Cancer Study Group. Surgical adjuvant therapy for stage II and III adenocarcinoma and large cell undifferentiated carcinoma. Chest. 1994; 106(Suppl):293S–296S.
10. Lad T, Rubinstein L, Sadeghi A. The Lung Cancer Study Group. The benefit of adjuvant treatment for resected locally advanced nonsmall cell lung cancer. J Clin Oncol. 1988; 6:9–17.
11. Feld R, Rubenstein L, Thomas P. The Lung Cancer Study Group. Adjuvant chemotherapy with cyclophosphamide, doxorubicin, and cisplatin in patients with completely resected Stage I nonsmall cell lung cancer. J Natl Cancer Inst. 1993; 85:299–306.
12. Niiranen A, Niitamo-Korhonen S, Kouri M, Assendelft A, Mattson K, Pyrhonen S. Adjuvant chemotherapy after radical surgery for nonsmall-cell lung cancer: a randomized study. J Clin Oncol. 1992; 10:1927–1932.

13. Keller S, Vangel MG, Adak S, et al. The influence of gender on survival and tumor recurrence following adjuvant therapy of completely resected stages II and IIIA nonsmall cell lung cancer. Lung Cancer. 2002; 37:303–309.

14. Ichinose Y, Tada H, Koike T, et al. A randomized phase III trial of postoperative adjuvant chemotherapy in patients with completely resected stage IIIA-N2 nonsmall cell lung cancer: Japan Clinical Oncology Group (JCOG) 9304 Trial. Proc ASCO. 2001; 20:A1241.
15. Alam N, Shepherd F, Winton T, et al. Compliance with post-operative adjuvant chemotherapy in nonsmall cell lung cancer An analysis of National Cnacer Institute of Canada and Intergroup trial JBR.10 and a review of the literature. Lung Cancer. 2005; 47:385–394.
16. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997; 111:1710–1717.

17. Rosell R, Felip E, Maestre J, et al. The role of chemotherapy in early nonsmall cell lung cancer management. Lung Cancer. 2001; 34:S63–74.
18. Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest. 1992; 101:1332–1337.

19. Wada H, Nakamura T, Nakamoto K, et al. Thirty-day operative mortality for thoracotomy in lung cancer. J Thorac Cardiovasc Surg. 1998; 115:70–73.

20. Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007; 83:1245–1250.

21. Martin J, Ginsberg RJ, Abdolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thor Surg. 2001; 72:1149–1154.

22. Albain KS, Scott CB, Rusch VR, et al. Phase III comparison of concurrent chemotherapy plus radiotherapy (CT/RT) and CT/RT followed by surgical resection for stage IIIA (pN2) nonsmall cell lung cancer: initial results from intergroup trial 0139 (RTOG 93–09). Proc Am Soc Clin Onc. 2003; 22:A2497.
23. Gridelli C, Perrone F, Gallo C, et al. MILES Investigators. Chemotherapy for elderly patients with advanced nonsmall cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) Phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–372.
Go to : 
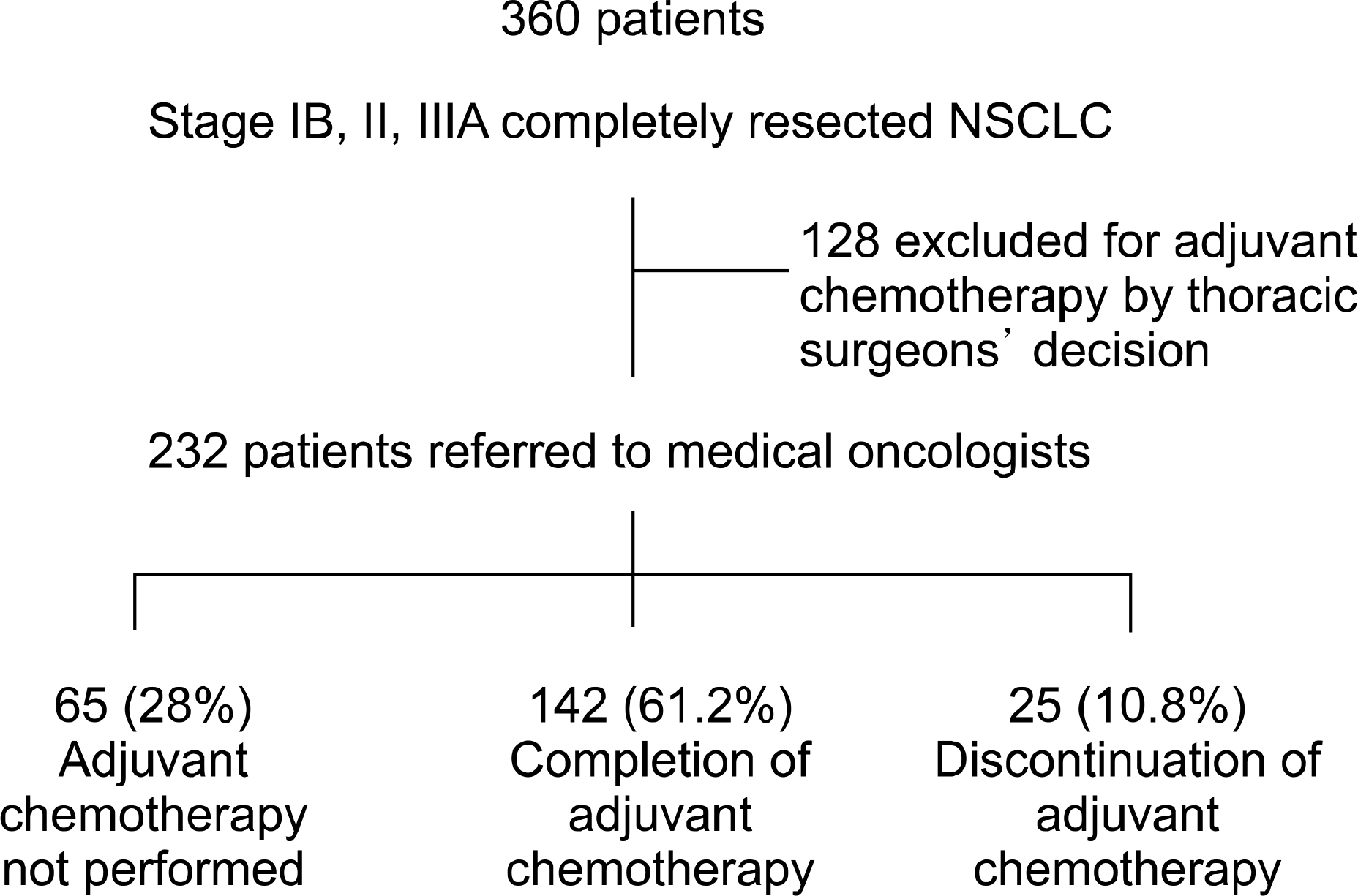 | Fig. 1.Adjuvant chemotherapy treatment flow from thoracic surgeons to medical oncologist. NSCLC: non-small cell lung cancer. |
Table 1.
Patient Characteristics (n=232)
| Variables | Number (%) |
|---|---|
| Male | 178 (76.7) |
| Operation procedure | |
| Lobectomy | 207 (89.2) |
| Pneumonectomy | 25 (10.8) |
| VATS* | 20 (8.6) |
| Histology | |
| Squamous cell carcinoma | 115 (49.6) |
| Adenocarcinoma | 95 (40.9) |
| Others | 22 (9.5) |
| Pathologic staging | |
| Stage IB | 81 (34.9) |
| Stage IIA | 8 (3.5) |
| Stage IIB | 59 (25.4) |
| Stage IIIA | 84 (36.2) |
| Chemotherapy start failure | 65 (28.0) |
| Chemotherapy regimen | |
| GemzarⓇ+Cisplatin (GP) | 79 (34.1) |
| TaxolⓇ+Cisplatin (TP) | 46 (19.8) |
| GemzarⓇ+Carboplatin (GC) | 4 (1.7) |
| TaxolⓇ+Carboplatin (TC) | 14 (6.0) |
| Navelbine+Cisplatin (NP) | 6 (2.6) |
| Irinotecan+Cisplatin (IP) | 5 (2.2) |
| Etoposide+Cisplatin (EP) | 6 (2.6) |
| Others | 7 (3.0) |
Table 2.
Univariate Analysis according to the Completion of Adjuvant Chemotherapy
| Variables | Completion (n=142) | Discontinuation (n=25) | p value | None (n=65) | p value |
|---|---|---|---|---|---|
| Age (year) | 59.0±8.9 | 59.4±10.9 | 0.847 | 65.4±6.8 | <0.001 |
| Sex (male/female) | 104/38 | 19/6 | 0.813 | 55/10 | 0.085 |
| Preop. PFT* | |||||
| FEV1 (liter)† | 2.37±0.65 | 2.52±0.51 | 0.283 | 2.27±0.56 | 0.144 |
| FEV1 (%) | 91.4±20.2 | 94.1±19.5 | 0.536 | 91.6±18.5 | 0.940 |
| FVC (liter)‡ | 3.26±0.71 | 3.39±0.69 | 0.358 | 3.19±0.76 | 0.380 |
| FVC (%) | 90.4±14.11 | 90.7±14.8 | 0.912 | 89.2±13.77 | 0.551 |
| Smoking | 100 | 15 | 0.350 | 53 | 0.071 |
| Pneumonectomy | 17 | 2 | 0.742 | 6 | 0.653 |
| Pathologic staging | 0.616 | 0.045 | |||
| IB | 43 | 5 | 1 | ||
| IIA | 7 | 0 | 18 | ||
| IIB | 38 | 8 | |||
| IIIA | 54 | 12 | |||
| Histology | 0.328 | 0.076 | |||
| Adenocarcinoma | 61 | 13 | 40 | ||
| Squamous cell ca. | 67 | 8 | 0.076 | ||
| Others | 14 | 4 | |||
| Operation time (minutes) | 192.3±59.5 | 210.6±92.1 | 0.346 | 205.4±57.6 | 0.266 |
| In-hospital stay (days) | 11.9±3.4 | 13.6±6.5 | 0.065 | 15.6±14.2 | 0.064 |
| VATS§ | 14 | 1 | 0.474 | 5 | 0.492 |
| Postoperative complications | 41 | 6 | 0.644 | 30 | 0.013 |
| Interval from surgery to | |||||
| chemotherapy (days) | 34.5±8.8 | 36.6±9.9 | 0.297 | – | – |
Table 3.
Multivariate Analysis of Adjuvant Chemotherapy
| Variables | p value | Odds ratio | 95% CI |
|---|---|---|---|
| Sex | 0.347 | 1.993 | 0.473∼8.393 |
| Age | <0.001 | 0.895 | 0.850∼0.943 |
| Operation time | 0.687 | 0.999 | 0.994∼1.004 |
| In-hospital stay | 0.136 | 0.949 | 0.885∼1.017 |
| Histology | 0.367 | 1.151 | 0.643∼2.06 |
| Postoperative | |||
| complications | 0.229 | 0.636 | 0.304∼1.33 |
| Smoking | 0.867 | 0.893 | 0.239∼3.342 |
| FEV1* | 0.761 | 0.903 | 0.469∼1.741 |
| Pathologic staging | 0.045 |
Table 4.
Stage Response and Toxicity in Adjuvant Chemotherapy Trials for NSCLC




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download