Abstract
Purpose
Cyclooxygenase-2 (COX-2) and its metabolite, PGE2 affect multiple tumorigenesis, including angiogenesis, invasion, and tumor-induced immune suppression. Their overexpression is association with impaired immune cell function in many tumors. Indoleamine 2,3-dioxygenase (IDO) is an emerging immunoregulatory enzyme that can catalyze the initial rate-limiting step in tryptophan catabolism, by causing tryptophan depletion can block T lymphocyte activation, and thus, enable tumor cells to escape from immune system. Although the potential of immunosuppression associated with tumor-produced COX-2 has been suggested, the mechanism of immunosuppression in tumor immunology is not yet well defined. Thus, we hypothesized that the tumor immunity of COX-2 could be partly due to IDO-dependent immune tolerance. To test this hypothesis, we evaluated IDO expression in cancer cells treated with selective COX-2 inhibitor.
Materials and Methods
The A549 human adenocarcinoma cell line, murine Lewis lung carcinoma (LLC) cell line and C57Bl/6 mice were used for in vitro and in vivo studies. In vitro studies, A549 cells were treated with various concentrations of COX-2 inhibitor (PTPBS) or PGE2. IDO enzyme activity and protein expression were checked by IDO enzyme activity assay and Western blotting. In vivo study, the 20 mice were randomized into normal control, LLC inoculated control, and low and high selective COX-2 inhibitor (celecoxib 25 or 250 mg/kg/day) treated LLL inoculated mice groups (n=5 per group). At one month, mice were sacrificed and tumor mass was isolated for quantification of IDO expression by immunohistochemical stain and western blotting.
Results
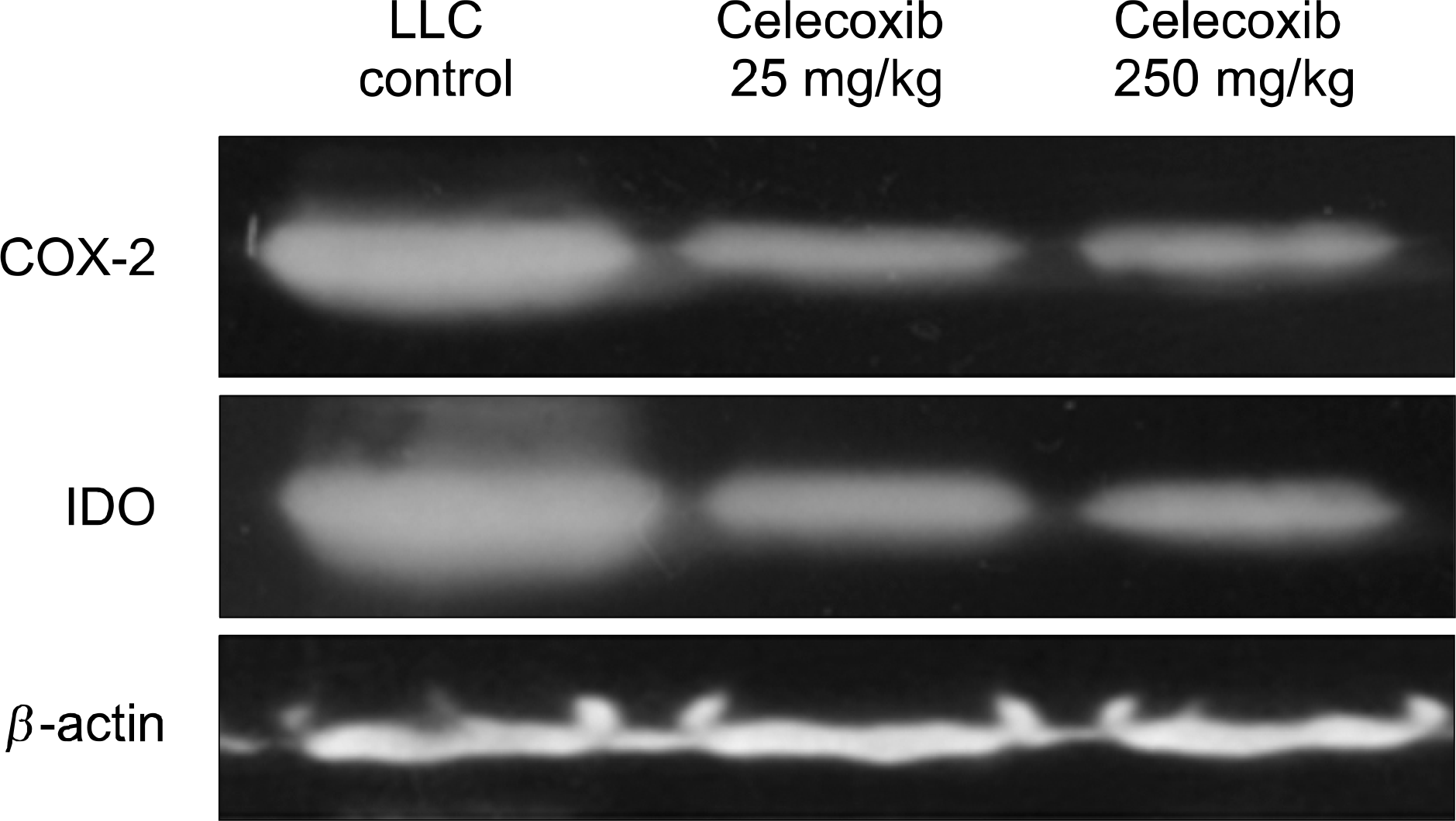
In vitro studies, PTPBS treated A549 cells showed a significant decreased in IDO enzyme activity and expression but PGE2 treated A549 cells showed increased in IDO expression. In vivo studies, the tumor mass and lung metastasis were attenuated by celecoxib (respectively, p<0.05, p<0.01). Compared with the LLC inoculated control group, mice treated with celecoxib had significant reductions in IDO expression of tumor mass (IDO immunohistochemical stain and western blotting).
Conclusion
The present study reveals that COX-2 inhibitor serves to restore the tumor-induced IDO expression and promotes antitumor reactivity in an immunocompetent murine lung cancer model. These findings further support the suggestion that COX-2 inhibitor is a potential pharmacological immunotherapy in cancer.
Go to : 
References
1. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995; 83:493–501.

2. Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000; 7:1163–1170.

3. Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: upregulation of interleukin 10 and downregulation of interleukin 12 production. Cancer Res. 1998; 58:1208–1216.
4. Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002; 62:632–635.
5. Sayama S, Yoshida R, Oku T, Imanishi J, Kishida T, Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 2,3-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. Proc Natl Acad Sci USA. 1981; 78:7327–7330.

6. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999; 189:1363–1372.

7. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000; 164:3596–3599.

8. Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988; 263:2041–2048.

9. Friberg M, Jennings R, Alsarraj M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002; 101:151–155.

10. Kudo Y, Boyd CA. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochim Biophys Acta. 2000; 1500:119–24.

11. Corbett TH, Griswold DP Jr, Roberts BJ, Peckham JC, Schabel FM Jr. Evaluation of single agents and combinations of chemotherapeutic agents in mouse colon carcinomas. Cancer. 1977; 40:2660–2680.

12. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991; 5:2516–2522.
13. Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER, Murphy MJ Jr. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994; 62:2277–2284.

14. Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004; 78:2632–2636.

15. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002; 9:1069–1077.

16. Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002; 196:447–457.
17. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003; 527:183–190.

18. Moffett JR, Espey MG, Namboodiri MA. Antibodies to quinolinic acid and the determination of its cellular distribution within the rat immune system. Cell Tissue Res. 1994; 278:461–469.

19. Yoshida R, Nukiwa T, Watanabe Y, Fujiwara M, Hirata F, Hayaishi O. Regulation of indoleamine 2,3-dioxygenase activity in the small intestine and the epididymis of mice. Arch Biochem Biophys. 1980; 203:343–351.

20. Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002; 168:3771–3776.

21. Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004; 19:319–326.

22. Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003; 9:12691274.

23. Dannenberg AJ, Altorki NK, Boyle JO, et al. Cyclooxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001; 2:544–551.

24. Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by inter-leukin-1 beta, tumor necrosis factoralpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res. 1998; 241:222–229.
25. Araki Y, Okamura S, Hussain SP, et al. Regulation of cyclo-oxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003; 63:728–734.
26. Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002; 9:28–35.

27. Sievers EM, Bart RD, Backhus LM, et al. Evaluation of cyclooxygenase-2 inhibition in an orthotopic murine model of lung cancer for dose-dependent effect. J Thorac Cardiovasc Surg. 2005; 129:1242–1249.

28. Diperna CA, Bart RD, Sievers EM, Ma Y, Starnes VA, Bremner RM. Cyclooxygenase-2 inhibition decreases primary and metastatic tumor burden in a murine model of orthotopic lung adenocarcinoma. J Thorac Cardiovasc Surg. 2003; 126:1129–1133.

29. Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000; 38:225–242.
Go to : 
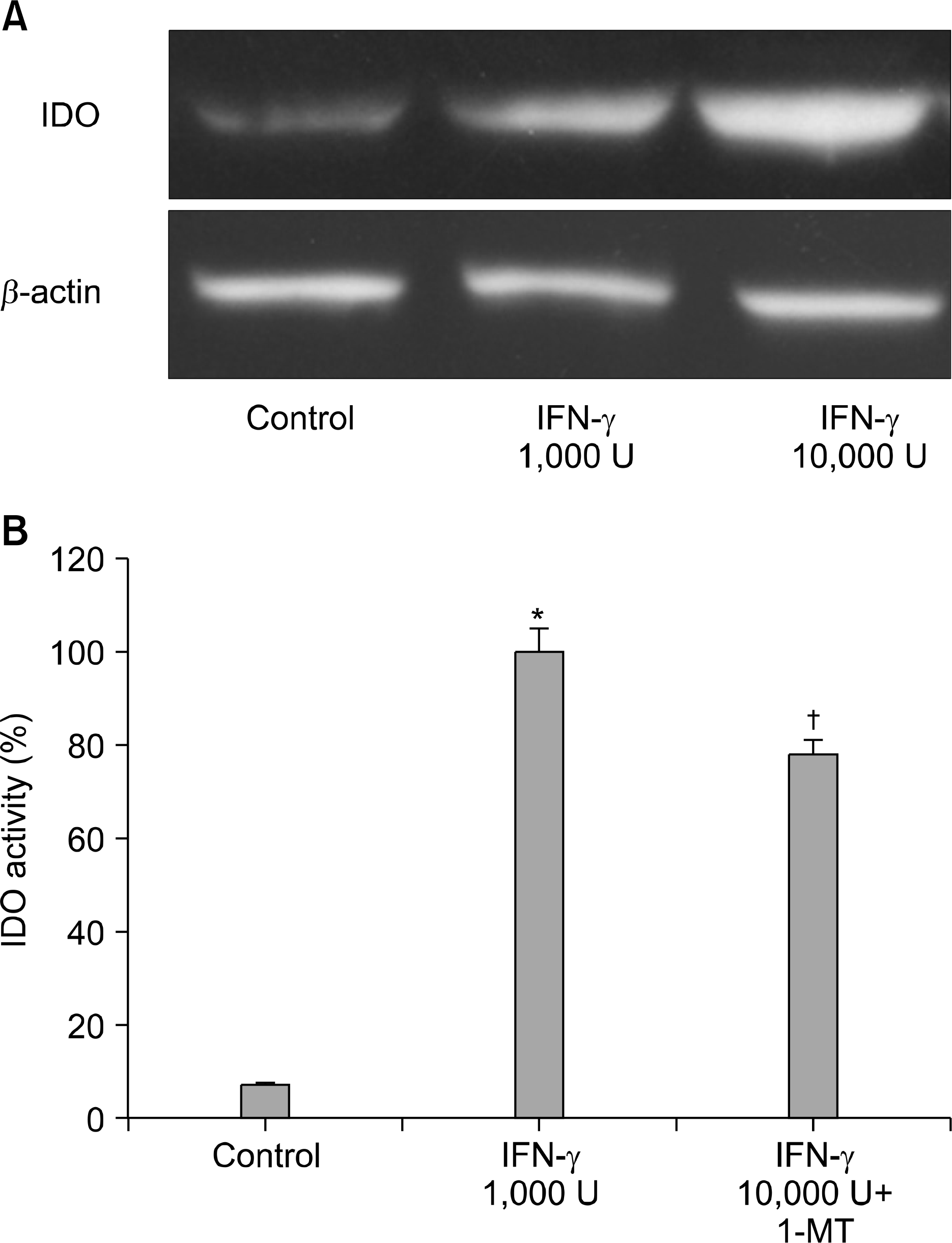 | Fig. 1.IDO expression in A549 cells stimulated with IFN-γ. (A) After incubating A549 cells with IFN-γ for 24 h, IDO expression was measured by Western blotting. (B) IDO activities were determined by measuring kynurenine formation and were calculated as percentages with respect to control. Data are means± SEM of three experiments (*p<0.01 vs. control, † p <0.05 vs. IFN-γ treated group). |
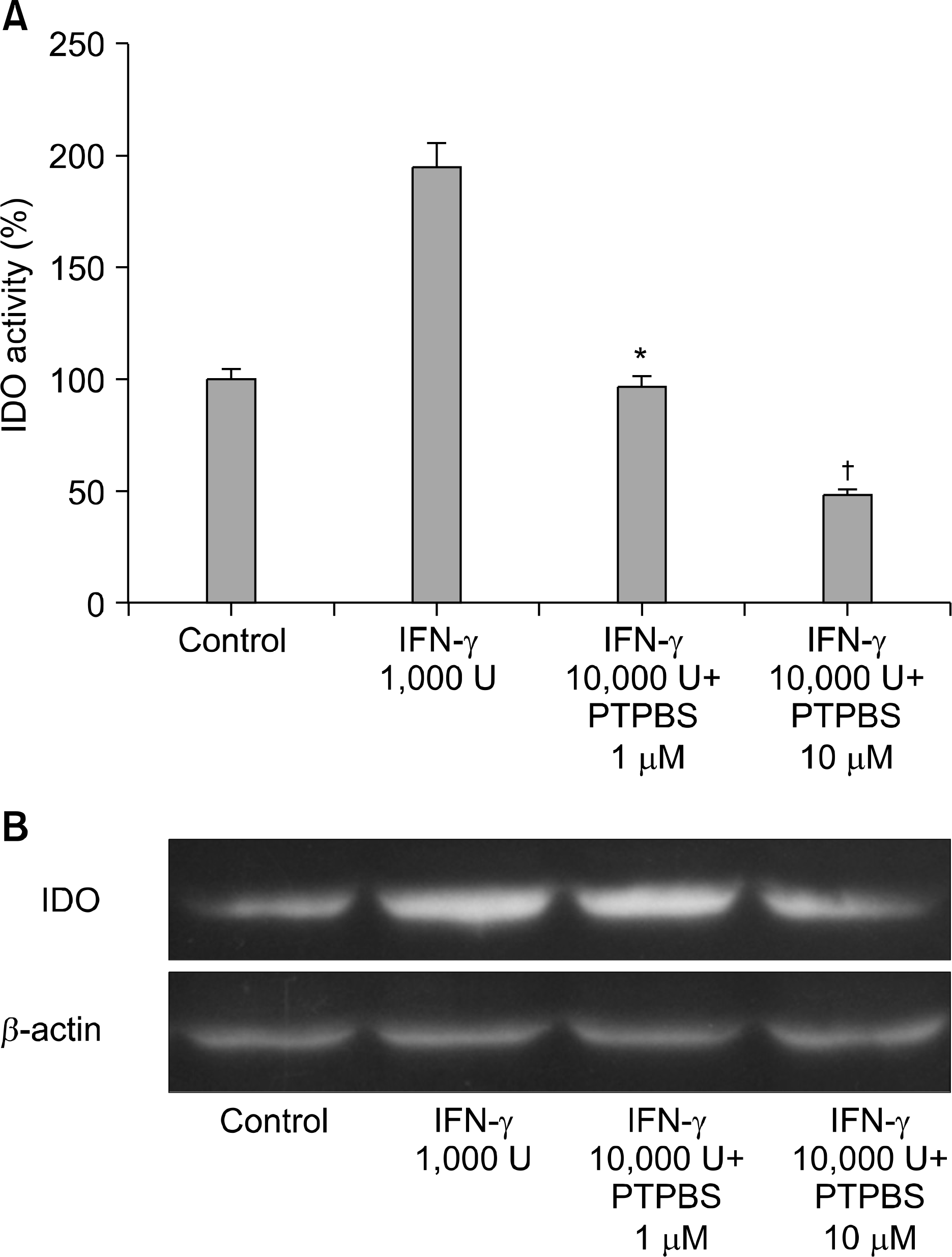 | Fig. 2.Effect of COX-2 inhibitor (PTPBS) on the activity of IDO produced by A549 cells in response to IFN-γ. (A) IDO activities were determined by measuring kynurenine formation and were calculated as percentages versus control. Data are means± SEM of three experiments (*p<0.01 vs. IFN-γ 1,000 U/ml, † p<0.05 vs. IFN-γ 1,000 U/ml+PTPBS 1μ M). (B) IDO expressions in A549 cells treated with IFN-γ plus PTPBS. |
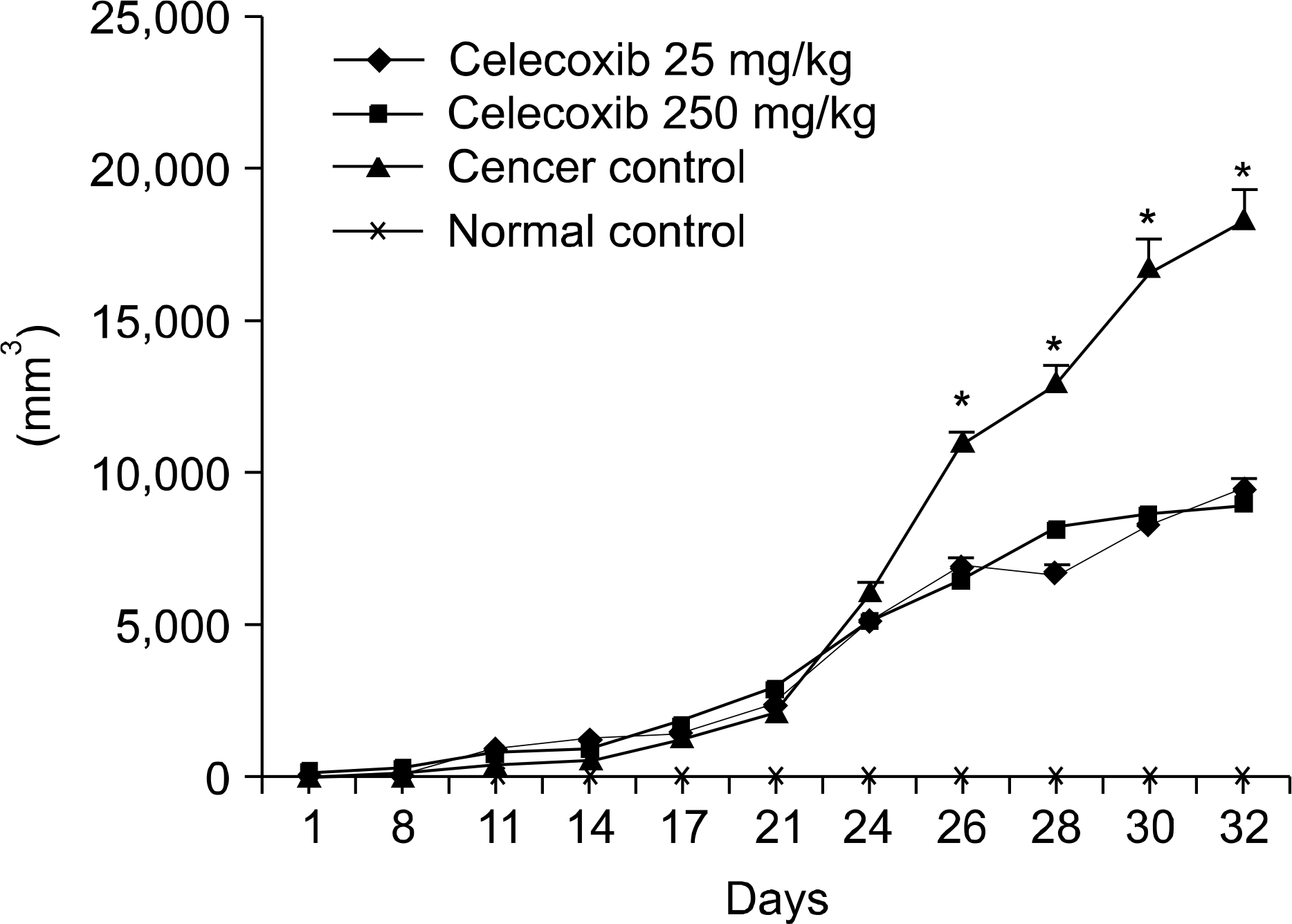 | Fig. 3.The growth curve of LLC tumors, in vivo. Tumors were measured in mice using a caliper in two perpendicular dia-meters, including the longest diameter and the shortest perpendicular diameter. A significant differences in tumor size was observed between the untreated LLC control group and celecoxib treated mice (*p<0.05 vs. celecoxib treated mice). |
 | Fig. 4.Histopathology of LLC induced lung metastasis (H&E stain, A: 40×, B: 100×). LLC cells were injected into C57Bl/6 mice as described in Methods, and mice were sacrificed 4 weeks after injection. Results were analyzed using the Mann-Whitney U test. (A) H&E staining revealed the pre-sence of LLC cells in metastatic sites. "T" indicates tumor tissue. The arrow indicates microscopic metastasis close to a blood vessel. (B) Lungs were analyzed microscopically for metastasis. Mice treated with celecoxib showed a significant decrease in the number of metastatic tumors (*p<0.01 vs. the untreated LLC control group). |
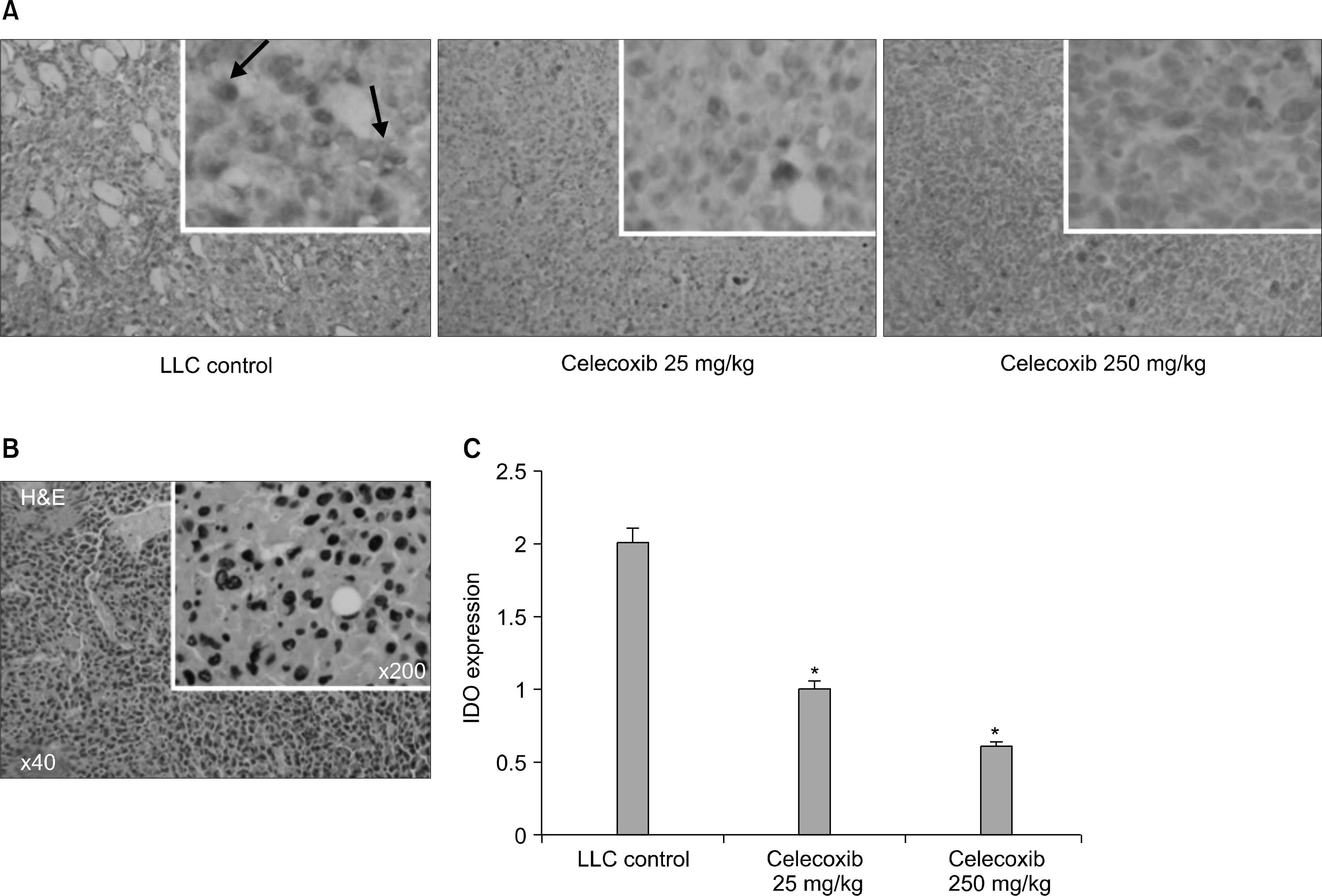 | Fig. 5.Immunohistochemical staining for IDO. Celecoxib treated mice showed significantly lower levels of IDO expression in tumor cells and tumor-infiltrating inflammatory cells. (A) Paraffin sections of isolated primary tumors were stained with anti-IDO antibody. Slides were counterstained with hematoxylin and visualized by light microscopy (at ×40 and ×200 magnification). Positively-stained cells are arrowed. (B) Photomicrograph of a tumor (H&E staining), showing original LLC cells in a tumor mass at its injection site. (C) IDO expression was determined by calculating the IDO stained area per microscopic field. IDO expression was significantly lower in celecoxib treatment group (*p<0.01 vs. the untreated LLC control group). However, no significant difference was observed between the celecoxib 25 mg and 250 mg groups. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download