Abstract
Purpose
Since the combination of cisplatin plus gemcitabine (CG) had a significant survival advantage for the treatment of patients with chemotherapy-naïve advanced or metastatic non-small cell lung cancer (NSCLC), CG combination have been evaluated with different schedules. However, the best schedule is still unclear. We designed to compare the efficacy and toxicity of CG combination chemotherapy in two different doses of gemcitabine (1,000 or 1,250 mg/m2 3-weekly).
Materials and Methods
We randomized patients with stage III or IV NSCLC into either gemcitabine 1,250 mg/m2 or gemcitabine 1,000 mg/m2. Patients received cisplatin 60 mg/m2 intravenously on day1 of each 3-week cycle. Gemcitabine was administered intravenously on days 1 and 8 of each 3-week cycle.
Results
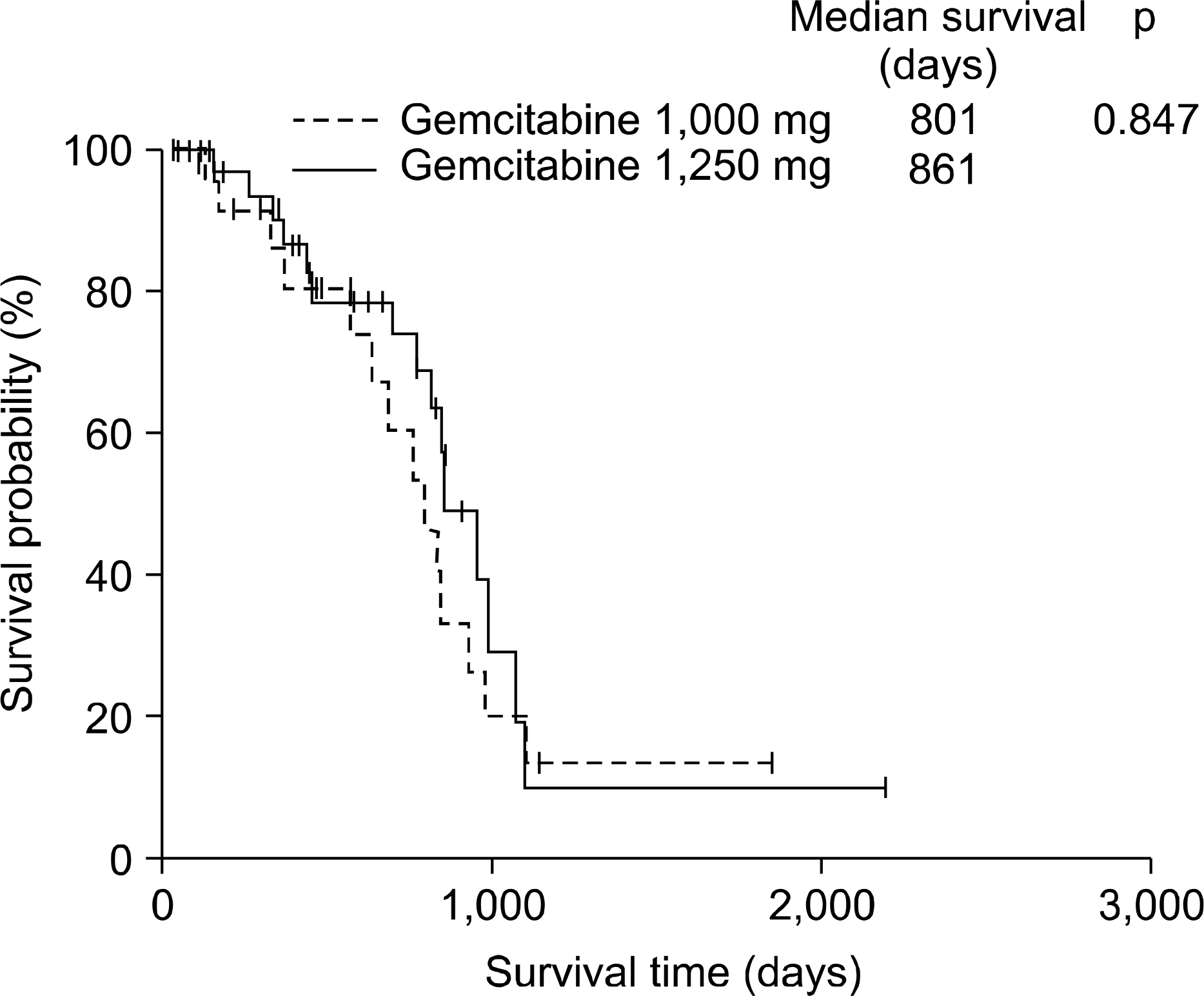
From April 2002 until July 2004, 125 patients were enrolled from four university hospitals (55 patients in the gemcitabine 1,000 mg/m2 arm and 70 patients in the gemcitabine 1,250 mg/m2 arm). Response rates were not significantly different in both arms (56.4% vs. 55.7%). However, grade 3 neutropenia was significantly lower in gemcitabine 1,000 mg/m2 arm compared to gemcitabine 1,250 mg/m2 arm (11.0% vs. 15.8%). No differences in non-haematologic toxicities in both arms except anorexia were observed. The median survival was 13.4 months for gemcitabine 1,000 mg group compared with 15.8 months for gemcitabine 1,250 mg group. There were no statistically significant differences in survival between the groups.
Go to : 
References
1. Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998; 48:6–29.

2. Sause W, Kolesar P, Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable nonsmall cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000; 117:358–364.
3. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in nonsmall cell lung cancer: a metaanalysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995; 311:899–909.
4. Abratt RP, Bezwoda WR, Goedhals L, Hacking DJ. Weekly gemcitabine with monthly cisplatin: effective chemotherapy for advanced nonsmall-cell lung cancer. J Clin Oncol. 1997; 15:744–749.

5. Shepherd FA, Cormier Y, Burkes R, et al. Phase II trial of gemcitabine and weekly cisplatin for advanced nonsmall cell lung cancer. Semin Oncol. 1997; 24:S8–27. -S8–30.
6. Anton A, Diaz-Fernandez N, Gonzalez Larriba JL, et al. Phase II trial assessing the combination of gemcitabine and cisplatin in advanced nonsmall cell lung cancer (NSCLC). Lung Cancer. 1998; 22:139–148.

7. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced nonsmall-cell lung cancer. N Engl J Med. 2002; 346:92–98.

8. Crino L, Scagliotti G, Marangolo M, et al. Cisplatin-gemcita-bine combination in advanced nonsmall-cell lung cancer: a phase II study. J Clin Oncol. 1997; 15:297–303.
9. Crino L, Scagliotti GV, Ricci S, et al. Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced nonsmall-cell lung cancer: a randomized phase III study of the Italian Lung Cancer Project. J Clin Oncol. 1999; 17:3522–3530.
10. Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic nonsmall-cell lung cancer. J Clin Oncol. 2000; 18:122–130.
11. Cardenal F, Lopez-Cabrerizo MP, Anton A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic nonsmall-cell lung cancer. J Clin Oncol. 1999; 17:12–18.

12. Rinaldi M, Crino L, Scagliotti GV, et al. A three-week schedule of gemcitabine-cisplatin in advanced nonsmall-cell lung cancer with two different cisplatin dose levels: a phase II randomized trial. Ann Oncol. 2000; 11:1295–1300.

13. Soto Parra H, Cavina R, Latteri F, et al. Three-week versus four-week schedule of cisplatin and gemcitabine: results of a randomized phase II study. Ann Oncol. 2002; 13:1080–1086.

14. Kim SY, Kim JS, Park HS. Screening of brain metastasis with limited magnetic resonance imaging (MRI). clinical implications of using limited brain MRI during initial staging for nonsmall cell lung cancer patients. J Korean Med Sci. 2005; 20:121–126.
15. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–181.

16. Steward WP, Dunlop DJ, Dabouis G, Lacroix H, Talbot D. Phase I/II study of gemcitabine and cisplatin in the treatment of advanced nonsmall cell lung cancer: preliminary results. Semin Oncol. 1996; 23:43–47.
17. Ostoros G, Szondy K, Gergely-Farnos E, et al. Efficacy of gemcitabine–cisplatin treatment in stage IIIA (“bulky”N2), IIIB and IV nonsmall cell lung cancer. Anticancer Res. 2005; 25:471–475.
Go to : 
Table 1.
Intracycle Dose Adjustments (Hematologic Toxicities)
| AGC*(×109/L) | Platelets (×109/L) | % of full-dose gemcitabine | |
|---|---|---|---|
| ≥1.0 | AND | ≥100 | 100 |
| 0.75 to 0.99 | OR | 75 to 99 | 75 |
| 0.5 to 0.74 | OR | 50 to74 | 50 |
| <0.5 | OR | <50 | HOLD |
Table 2.
Patients Characteristics
| Gemcitabine (1,000 mg/m2)(n=55) | Gemcitabine (1,250 mg/m2) (n=70) | |
|---|---|---|
| No. of patients (%) | No. of patients (%) | |
| Age (years) | ||
| Median | 64 | 65 |
| Range | 40∼76 | 30∼78 |
| Sex | ||
| Male | 47 (85.5) | 58 (82.9) |
| Female | 8 (14.5) | 12 (17.1) |
| ECOG | ||
| 0 | 8 (14.5) | 15 (21.4) |
| 1 | 38 (69.1) | 46 (65.7) |
| 2 | 9 (16.4) | 9 (12.9) |
| Stage* | ||
| IIIA | 17 (30.9) | 11 (15.8) |
| IIIB | 20 (36.4) | 31 (44.2) |
| IV | 18 (32.7) | 28 (40.0) |
| Histology | ||
| Adenocarcinoma | 20 (36.4) | 29 (41.4) |
| Squamous cell | 32 (58.2) | 34 (48.6) |
| Large cell | 2 (3.6) | 1 (1.4) |
| Others | 1 (2.8) | 6 (8.6) |
Table 3.
Comparision of Response between Two Groups
Table 4.
Comparison of Hematologic Toxicities between Two Groups
| Gemcitabine (1,000 mg/m2)(n*=480) | Gemcitabine (1,250 mg/m2) (n*=618) | p | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Granulocytopenia | |||||
| Total | 53 | 11 | 98 | 15.8 | 0.016 |
| Grade 3 | 51 | 10.6 | 84 | 13.5 | |
| Grade 4 | 2 | 0.4 | 14 | 2.3 | |
| Thrombocytopenia | |||||
| Total | 22 | 4.6 | 23 | 3.7 | 0.113 |
| Grade 3 | 20 | 4.1 | 20 | 3.2 | |
| Grade 4 | 2 | 0.5 | 3 | 0.5 | |
| Anemia | |||||
| Total | 29 | 6.0 | 42 | 6.8 | 0.019 |
| Grade 3 | 29 | 6.0 | 42 | 6.8 | 0.119 |
| Grade 4 | 0 | 0.0 | 0 | 0.0 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download