Abstract
Purpose
Overexpression of COX-2t an enzyme responsible fro the synthesis of prostaglandins, is well linked to human chronic lung diseases. The mechanism by which COX-2 expression is increased or enhanced in cancer cells remains largely unknown. Any compound which can reduce COX-2 expression may be considered as an anti-cancer agent.
Materials and Methods:
Leptomycin Β (LMB) is a metabolite of Streptomyces and a specific inhibitor of CRM1 nuclear export receptor A549 is a human lung cancer cell line. To evaluate the effect of LMB on COX-2 expression induced by IL-1 β, a pro-inflammatory cytokine, in A549 cells, Western blot and RT-PCR assays were applied to measure COX-2 protein and mRNA expressions in response to IL-1 β, respectively. Luciferase experiments were done to measure promoter activity of COX-2, NF- a B or AP-1. CRM1 siRNA trasfection experiment was performed to knock-down endogenous CRM! Biochemical protein fractionation method was also carried out to see intracellular localization of proteins.
Results:
LMB at 9 nM strongly suppressed IL-1 β-induced expression of COX-2 protein that was attributable to decreased COX-2 transcript and promoter activity, but not mRNA stability. Distinctly, knock-down of CRM1 had no effect on COX-2 expression by IL-1 β. Moreover, LMB did not affect IL-1 -induced phosphorylation of ERK-1/2, JNK- 1/2, and ρ3δ MAPK or AP-1 promoter activity. In contrast, LMB blocked IL-1 β- mediated cytosolic Ia:B-« degradation, p65 NF-a-B nuclear translocation, and NF-a:B promoter activity.
REFERENCES
1.Smith WL: DeWitt DL., Garavito R. Cyclooxygenases: structural, cellular, and molecular biology, Annu Rev Biochem. 2000. 69:145–182.
2.Mamett LJ., DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002. 42:55–80.
3.Hla T., Bishop-Bailey D: Liu CH., Schaefers HJ: Trifan OC. Cyclooxygenase-1 and -2 isoenzymes, Int J Biochem Cell Biol. 1999. 31:551–557.
4.Hawk ET., Viner JL., Dannenberg A., DuBois RN. COX-2 in cancer-a player that's defining the rules. J Natl Cancer Inst. 2002. 94:545–546.

5.Herschman HR., Reddy ST., Xie W. Function and regulation of prostaglandin synthase-2. Adv Exp Med Biol. 1997. 407:61–66.

6.Newton R: Kuitert LM: Bergmann M., Adcock IM: Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX: 2 gene expression by IL-lbeta. Biochem Biophys Res Commun. 1997. 237:28–32.
7.Inoue H., Yokoyama C., Ham S., Tone Y., Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995. 270:24965–24971.
8.Ristimaki A., Garfinke】S: Wessendorf J., Maciag T., Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994. 269:11769–11775.
9.Srivastava SK., Tetsuka T., Daphna-Iken D: Morrison AR. IL-1 beta stabilizes COX II mRNA in renal mesangial cells: role of 3'-untranslated region. Am J Physiol. 1994. 267:504–508.

10.Jang BC., Munoz-Najar U., Paik JH., Claffey K., Yoshida M., Hla T. Leptomycin B, an inhibitor of the nuclear export receptor CRM1: inhibits COX-2 expression. J Biol Chem. 2003. 278:2773–2776.

11.Chen W: Tang Q., Gonzales MS., Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001. 20:3921–3926.

12.Hunot S: Vila M: Teismann P, et al. JNK-mediated induction of cyclooxygenase-2 is required for neurodegeneration in a mouse model of Parkinson's disease, Proc Natl Acad Sci USA. 2004. 101:665–670.
13.Hamamoto T: Gunji S., Tsuji H: Beppu T. Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J Antibiot (Tokyo). 1983. 36:39–45.
14.Yoshida M., Nishikawa M., Nishi K: Abe K: Horinouchi S., Beppu T. Effects of leptomycin B on the cell cycle of fibro-Wasts and fission yeast cells. Exp Cell Res. 1990. 187:150–156.
15.Jang BC., Paik JH., Jeong HY, et al. Leptomycin B-induced apoptosis is mediated through caspase activation and down-regulation of Mcl-1 and XIAP expression, but not through the generation of ROS in U937 leukemia cells. Biochem Pharmacol. 2004. 68:263–274.

16.Lecane PS., Kiviharju TM: Sellers RG., Peehl DM. Leptomycin B stabilizes and activates p53 in primary prostatic epithelial cells and induces apoptosis in the LNCaP cell line. Prostate. 2003. 54:258–267.

17.Komiyama K: Okada K., Tomisaka S: Umezawa I: Hamamoto T: Beppu T. Antitumor activity of leptomycin B. J Antibiot (Tokyo). 1985. 38:427–429.
18.Nishi K., Yoshida M., Fujiwara D., Nishikawa M., Horinouchi S: Beppu T. Leptomycin B targets a regulatory cascade of crml, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994. 269:6320–6324.
19.Fomerod M: Ohno M: Yoshida M., Mailaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997. 90:1051–1060.

20.Brennan CM., Gallouzi IE., Steitz JA. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J Cell Biol. 2000. 151:1–14.

21.Giannini A., Mazor M: Orme M: Vivanco M., Waxman J: Kypta R. Nuclear export of alpha-catenin: overlap between nuclear export signal sequences and the beta-catenin binding site. Exp Cell Res. 2004. 295:150–160.
22.Huang TT: Kudo N., Yoshida M: Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of Ikappa-Balpha controls cytoplasmic localization of inactive NF-kappa B/1 kappa Bal pha complexes∗ Proc Natl Acad Sci. 2000. 97:1014–1. 이9.
23.Jang BC., Sung SH., Park JG, et al. Leptomycin B, a metabolite of Streptomyces, inhibits the expression of inducible nitric oxide synthase in BV2 microglial cells. Int J Oncol. 2006. 29:1509–1515.

24.Simeonidis S: Stauber D., Chen G., Hendrickson WA: Thanos D. Mechanisms by which IkappaB proteins control NF-kappaB activity. Proc Natl Acad Sci. 1999. 96:49–54.
25.Liu W., Reinmuth N., Stoeltzing O, et al. Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res. 2003. 63:3632–3636.
26.Sawano H., Haneda M: Sugimoto T., Inoki K., Koya D., Kikkawa R. 15-Deoxy-Deltal2∗ 14-prostaglandin J2 inhibits IL-1 beta-induced cyclooxygenase-2 expression in mesangial cells. Kidney Int. 2002. 61:1957–1967.
27.Erkmann JA., Kutay U. Nuclear export of mRNA: from the site of transcription to the cytoplasm. Exp Cell Res. 2004. 296:12–20.

28.Rodriguez MS., Thompson J: Hay RT., Dargemont C. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J Biol Chem. 1999. 274:9108–9015.
29.Han Y., Weinman S: Boldogh I: Walker RK: Brasier AR. Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. J Biol Chem. 1999. 274:787–794.
Fig. 1.
Effect of ᄂMB on Iᄂ-1 㽐-induced COX-2 protein and mRNA and promoter activity in A549 lung cancer cells, (Α, Β) A549 celts were pre-treated with the indicated concentrations of I一MB for 1 h. Cells were then exposed to IL-1 β for 4 h. Total cell lysates and RNA were prepared, and analyzed for COX-2 immunoblot (A) and RT-PCR (B), respectively. Actin or GAPDH was used to evaluate the relative expression of COX-2 protein or mRNA, (C) A549 cells were transfected with COX-2 promoter/luciferase DNA along with control pRL-TK DNA for 24 h and then exposed to IL-1 for 4 h in absence or presence of LMB, Cell lysates were prepared and used for reporter gene activity. Data are mean 士 S.E. of three independent experiments.
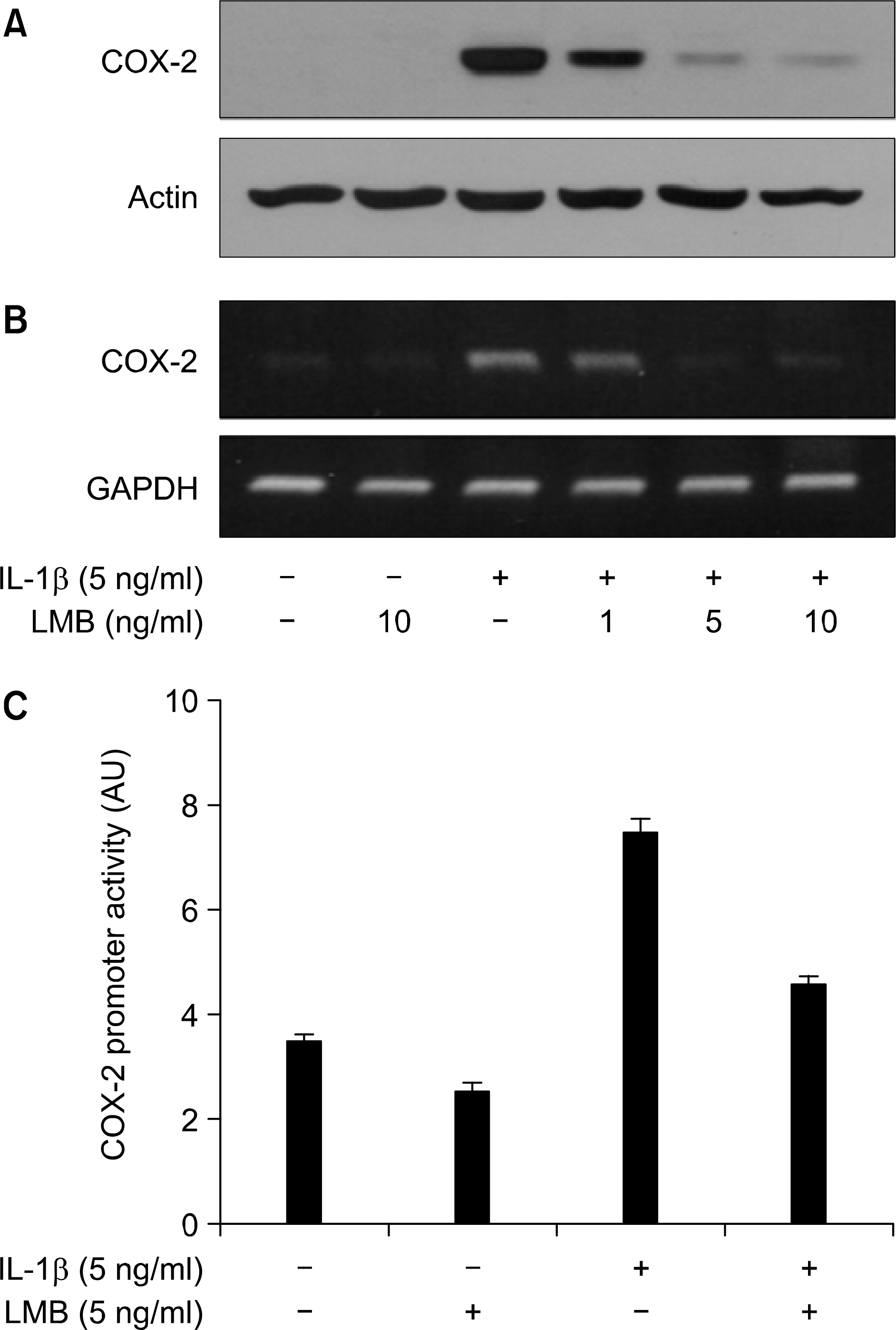
Fig. 2.
Effect of LMB on IL-1 -induced proteolysis of U-Β-α and NF-/cB nuclear localization and its promoter activity in A549 cells. (A) A549 cells were pre-treated with LMB for 1 h. Cells were then exposed to L-0 for 05 h in absence or presence of LMB. Total cell lysates were prepared, and used to measure total protein of ϊκΒ-α, p65 NF-λ’ΒôHuRôor actin by immunoblot with respective antibody. NS indicates nonspecific protein band. (B) Cytosolic and η䴸clear proteins were prepared and used to measure the level of cytosolic κΒ-α and nuclear p65 NF-^B by immunoblot with respective antibody. (C) A549 cells were transfected with NF-a:B promoter/luciferase DNA along with control pRL-TK DNA for 24 h and then exposed to IL- 1 for 4 h in absence or presence of LMB. Cell lysates were prepared and used for reporter gene activity. Data are mean 土 S.E of three independent experiments. (D) The same as in (C) except HuR immunoblot.
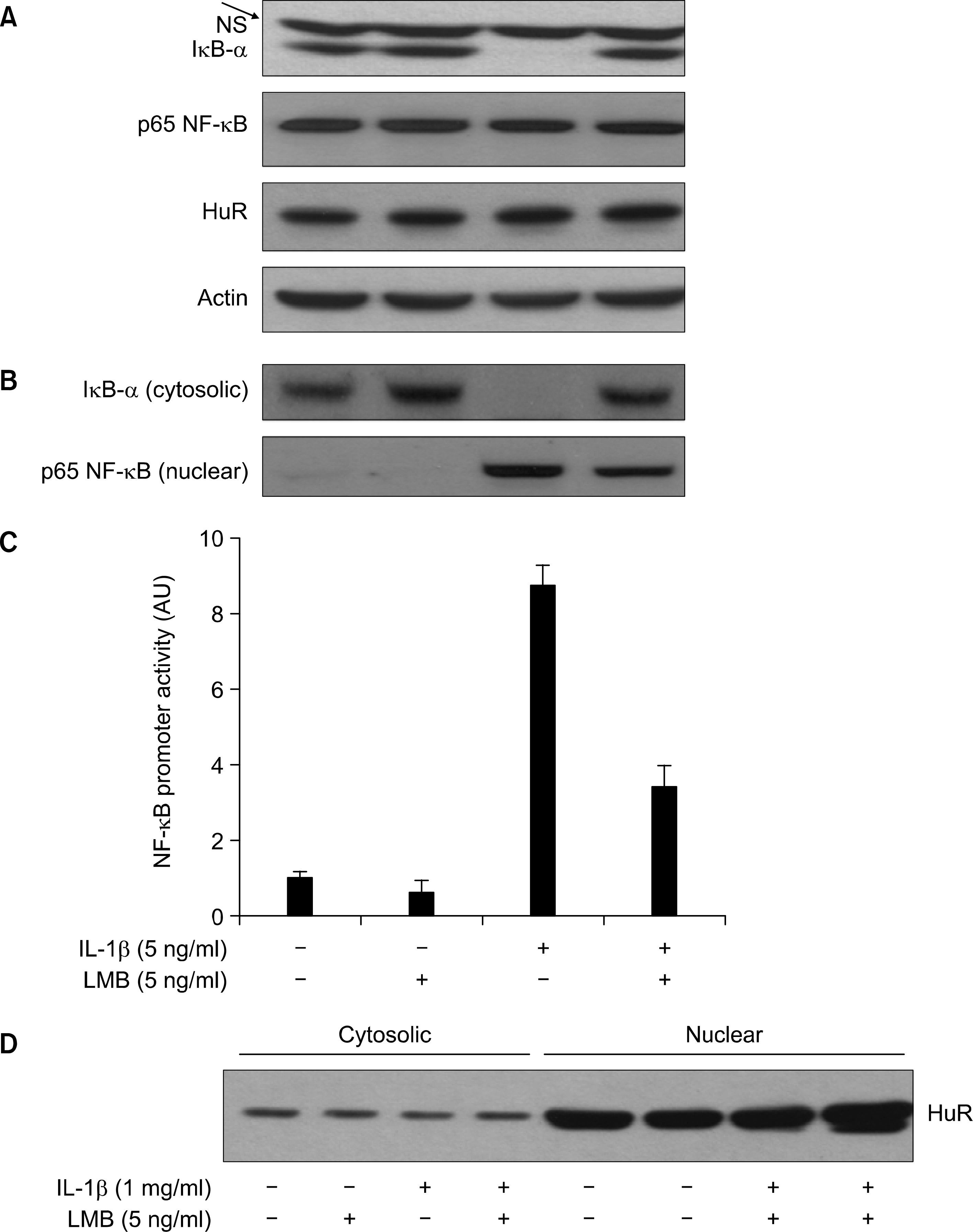
Fig. 3.
Effect of LMB on IL-1 㻐-mediated activation of MAPKs and AP-1 promoter activity in A549 cells, (A) A549 cells were pre-treated with LMB for 1 h. Cells were then exposed to IL-1 β for 0.5 h in absence or presence of LMB, Total cell tysates were prepared, and used to measure the extent of phosphorylation of ERK-1/2, JNK-1/2, or p38 MAPK. Total protein level of each protein was confirmed with striping and reprobing the membrane by immunoblot 䴸sing respective antibody, (Β) A549 cells were transfected with AP-1 promoter/luciferase DNA along with control pRL-TK DNA for 24 h and then exposed to IL-1 㻐 for 4 h in absence or presence of LMB. Cell lysates were prepared and used for reporter gene activityôData are mean 士 SôE. of three independent experiments.
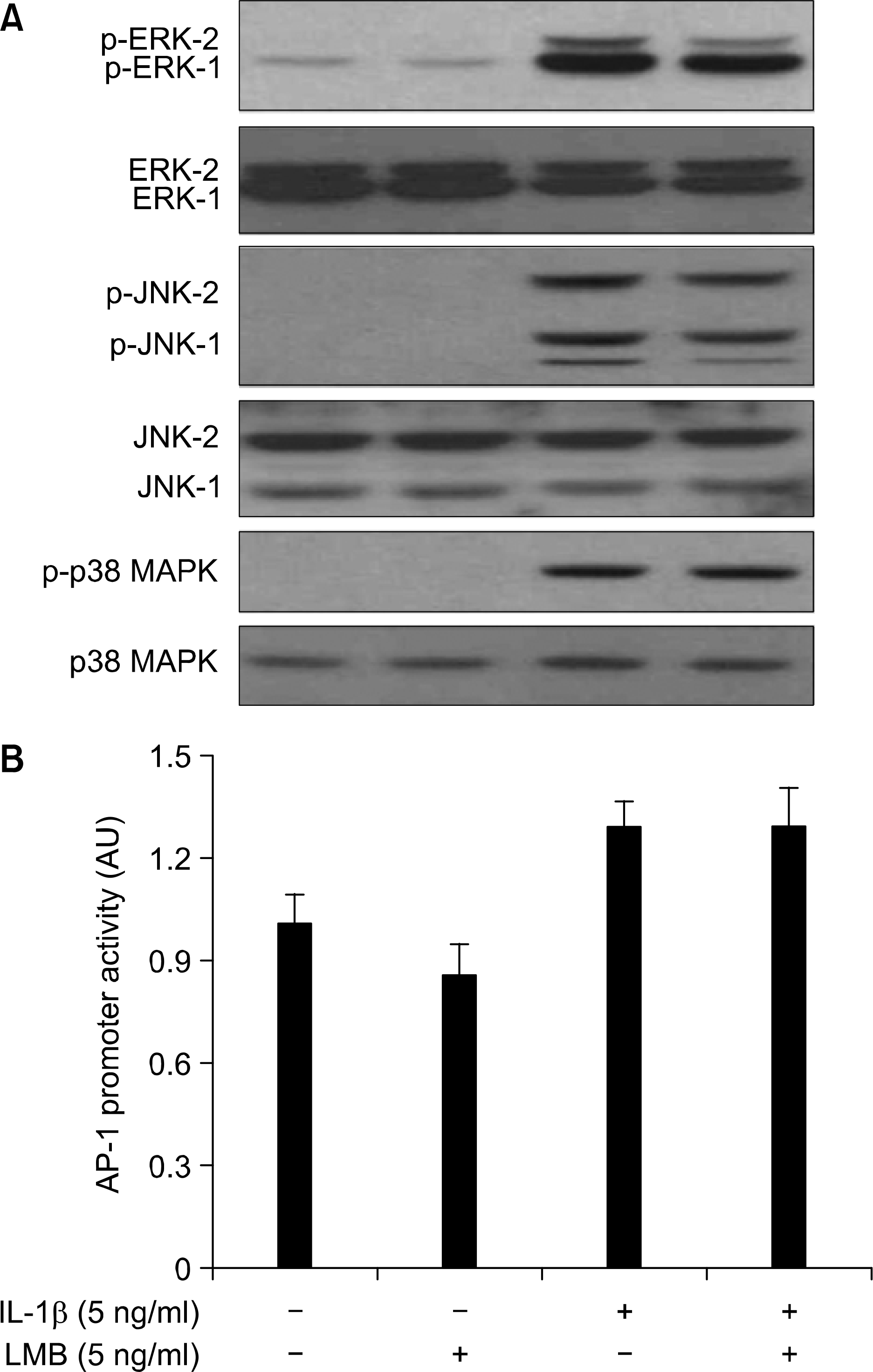
Fig. 4.
No dependence of CRM1 and COX-2 mRNA stability in ᄂMB inhibition of IL-1 -induced COX-2 expression in A549 cells. (A) A549 cells were transfected with 80 nM of control or CRM1 siRNA. Cells were then exposed to IL-1 β in absence or presence of LMB for 4 h. Cell lysates were prepared and used to measure the expression level ᄋf CRM 1ôCOX-2, or actin by immunoblot with respective antibody. (Β) A549 cells were primarily treated without (lane 1) or with IL-1 㽐 (lanes 2〜 10) for 4 h to highly induce COX-2 mRNA. Cells were then exposed to Iᄂ-1/3 in the presence of actinomycin D (Act D)’ a transcription inhibitor, (lanes 3 〜 10) in absence (lane 3-6) or presence (lanes 7-10) of LMB for the indicated times. At each time, total RNA was prepared, and 䴸sed for COX-2 or GAPDH RT- PCR to measure the amount of respective RNA remained in the cells.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download