Abstract
Radiation therapy is one of the most important therapeutic modalities for the treatment of lung cancer. Radiation pneumonitis is one of the important complications associated with radiotherapy for lung cancer. Radiation pneumonitis is generally limited to the irradiated lung and is manifested by the insidious onset of dry cough, dyspnea, and mild fever, resulting in damage and edematous changes of alveolar structures on histologic inspection. Clinically, diffuse bilateral radiation pneumonitis accompanied with acute symptoms after unilateral thoracic irradiation appears very rarely. Histopathologic examinations for the diagnosis of out-of-field radiation pneumonitis are rarely performed. We herein describe a case of extensive bilateral radiation pneumonitis which developed acutely after salvage radiotherapy for squamous cell carcinoma in the left upper lobe of the lung. The condition was confirmed by a diagnostic help of histopathologic findings.
Radiation-induced lung injury is a complication caused by radiation treatment (RT) of thoracic malignancies and is manifested by an early acute phase of radiation pneumonitis (RP) and a late phase of pulmonary fibrosis. RP occurs 1 to 3 months after completion of a radiotherapy course and is manifested by the insidious onset of dry cough, dyspnea, and mild fever. RP is generally self-limiting in its course (1,2). RP appears to be generally confined to the irradiated field and seldom occurs beyond the irradiated field (3-5). Extensive diffusion of RP in both lung fields is very rare, but the case was manifested by the acute onset of dyspnea within 1 month from completion of RT.
RP is diagnosed clinically after ruling out other pulmonary diseases. Histopathologic examinations for the diagnosis of out-of-field RP are rarely performed (2).
We herein describe a case of extensively diffuse bilateral RP which developed 3 weeks after completion of salvage RT targeted to the primary remnant non-small cell lung cancer (NSCLC) of the left upper lobe (LUL). The diagnosis was confirmed with a diagnostic help of histopathologic findings of the contralateral lung tissue.
A 64-year-old man visited for dyspnea and dry cough which started 3 weeks after completion of RT. He had been diagnosed with stage IV (T3N2M1a) squamous cell carcinoma of a 9 cm sized lung mass in LUL with metastatic mediastinal lymph nodes and an ipsilateral metastatic pleural nodule. He had received 5 cycles of systemic combination chemotherapy with cisplatin and paclitaxel over the duration of 15 weeks. After 5 cycles of chemotherapy, the size of the lung mass decreased and the metastatic pleural nodule disappeared. Sequentially, he received salvage RT for the primary mass of LUL in a total dose of 72 Gy in 1.8 Gy daily fractions over 8 weeks, which was composed of 3-dimensional conformal radiation therapy (3,960 cGy) followed by Rapid-Arc radiotherapy (3,240 cGy) (Fig. 1). The mean radiation dose of the lung was 12.4 Gy and V20, which means that the percentage of lung volume receiving at least 20 Gy was only 24.6%. He had a 30-pack-year history of cigarette smoking and did not have any other illness except chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 2) and essential hypertension.
The patient appeared acutely ill. His blood pressure was 153/92 mm Hg, respiratory rate was at 24 breaths per minute, pulse rate was at 100 beats per minute and body temperature reached 37.9℃. His lung sounds revealed coarse crackles over both lung fields.
The peripheral blood count results were as follows: white blood cell count of 9,080/mm3 (neutrophil 83.2%, lymphocyte 10.2%, monocyte 4.5%, and eosinophil 1.9%), hemoglobin of 14.4 g/dL, and platelet count of 167,000/mm3. The C-reactive protein level was 16.6 mg/dL. The arterial blood gas analysis at room air revealed pH 7.480, pCO2 29.8 mm Hg, pO2 46.7 mm Hg, HCO3- 22 mmol/L, and O2 saturation of 85%. Blood chemistries showed that the blood urea nitrogen/creatinine level was at 21/1.13 mg/dL and the serum sodium, potassium, and chloride levels were at 137, 4.4, and 98 mmol/L, respectively. Cardiac biomarkers, tropnin T and creating kinase-MB, were within the normal range. Quantitative tests of anti-neutrophil cytoplasmic antibody and anti-nuclear antibody were all negative at 1:40 ratio. Rheumatic factor was at 1.8 IU/mL and anti-cyclic citrullinated peptide antibody was at 0.9 U/mL. No pathogen was grown in his sputum and 2 pairs of blood culture.
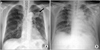
Simple chest radiograph (Fig. 2A) showed newly developed multifocal patchy infiltrative lesions in both lung fields compared with previous chest radiography 3 weeks ago (Fig. 2B). Chest computed tomography (CT) scans showed multifocal non-segmental ground glass opacities that were more predominent in the peripheral zone at both whole lung fields with partial interlobular septal thickening and no interval change of the known LUL mass (Fig. 3).
Bronchoscopic examination revealed no endobronchial lesions. Broncho-alveolar lavage (BAL) was performed in the lateral basilar segment of the right lower lobe (RLL), and trans-bronchial lung biopsy (TBLB) was performed in the posterior basilar segment of the RLL. The cell count results of BAL fluids showed a white blood cell count of 384/mm3 (neutrophil 12%, lymphocyte 24%, macrophage 64%, and eosinophil 0%) and red blood cell of 4,000/mm3. No bacteria and fungus were grown. A polymerase chain reaction test of Pneumocystis jirovescii was negative and cytology showed no malignant cells. The histology of the TBLB specimen revealed a bizarre, enlarged hyper-chromatic type II alveolar lining cell containing a prominent nucleoli (Fig. 4A), edema and thickening of alveolar walls, and accumulations of alveolar macrophages in the alveolar space (Fig. 4B).
Under the diagnosis of RP, we began to administer methyl-prednisolone 75 mg. However, his severe respiratory distress required steroid pulse therapy (methyl-prednisolone 1,000 mg daily for 3 days). Following this, his hypoxic status showed a wax and wane pattern. After 19 days, hospital-acquired pneumonia was developed to be combined with RP in both lower lung fields and his respiratory distress worsened, resulting in acute respiratory failure. After 23 days, the respiratory failure did not improve which led to cardiac arrest and the patient expired.
RT is used as one of the most important modalities for the treatment of lung cancer especially as the primary local therapy for patients with medically inoperable or unresectable NSCLC or as a palliative modality for patients with incurable NSCLC. RT is known to be followed by several complications such as radiation lung injury and esophageal injury. Radiation-induced lung injury is manifested by two distinct clinical stages: an early acute stage characterized by RP and a later chronic stage characterized by radiation fibrosis. RP is generally limited to the irradiated lung parenchyma, and radiographic changes are generally confined to the field of irradiation. RP presents with an insidious onset of dyspnea, cough, and mild fever 1 to 3 months after completion of RT. Although there have been reports on the occurrence of extensive RP outside the treatment portals (3-5), it is clinically very rare to have RP in both lung fields accompanied with a sudden onset of dyspnea within 1 month after completion of RT, especially salvage radiotherapy.
A patient who has received RT for lung cancer and complains of dyspnea or other respiratory symptoms should undergo chest CT scanning. CT scans are more useful in the diagnosis of RP rather than simple chest X-rays. However, chest CT scans of RP typically reveal interstitial infiltrates and/or a ground-glass appearance (6), which can be very difficult to distinguish from infection and interstitial lung disease (ILD). Thus, RP is confirmed after ILD, infection, and progression of known lung cancer are ruled out. In the present case, chest CT scans revealed multifocal ground glass opacities in both lung fields. We confirmed RP finally through bronchoscopic examination followed by BAL and TBLB after excluding pulmonary infection and progression of NSCLC.
Radiation-induced histologic changes of human lung tissues have been rarely reported because both surgical resections and autopsies after RT have seldom been performed (2). In experimental models, histopathologic findings of lung tissue after radiation injury showed loss of type I pneumocytes, enlargement and atypia of type II pneumocytes, increased exudates in alveolar spaces, edematous changes of the alveolar wall, aggregation of alveolar macrophages, and infiltration of the intersititium by monocytes and fibroblasts (7,8). However, it has not been elucidated that these changes are similarly develop in areas outside of the irradiated field, especially in the contralateral lung tissue after unilateral irradiation. In the present case, although TBLB was performed, contra-lateral lung tissues outside of the irradiation field were obtained, and histologic examination showed similar findings to the results of experimental models after radiation injury. These findings may suggest that RP that develops in bilateral lung fields show similar histopathologic changes as with in-field RP.
In the studies associated with RP outside of the irradiated field, BAL fluid analysis showed an increase of lymphocyte count (3,9). More specifically, a study showed a case of lymphocytic pneumonitis in the contra-lateral lung field after unilateral thoracic irradiation (9). Although the cell profile of BAL fluid shows mild lymphocytosis in the present case, there are many different opinions regarding this issue and it is not yet concluded that lymphocytic pneumonitis is related to RP developed outside of the irradiated field (2).
Higher dose of irradiation is generally used for salvage RT regimens. Significant lung injury from RT tends to depend on the total dose of irradiation, and radiation-induced lung damage is nearly common with doses in excess of 60 Gy. A small sized study suggested that irradiation with a higher mean lung dose is associated with the development of severe RP (10). However, other studies on risk factors associated with severe RP did not support this idea, especially involving bilateral lung fields (11-13). Further studies on risk factors for severe RP, with a special focus on bilateral lung fields, are needed in the future.
In conclusion, the present case suggests that though RT is confined to a part of the unilateral lung, RP may occur in a pattern of diffuse bilateral involvement of the whole lung at a comparatively early stage with similar histopathologic changes of RP in the contralateral lung to those of in-field RP.
Figures and Tables
Fig. 2
(A) Known lung cancer mass (arrow) in the left upper lobe. (B) Multifocal patchy infiltrative lesions in both lung fields.
References
1. Pastores SM, Voigt LP. Acute respiratory failure in the patient with cancer: diagnostic and management strategies. Crit Care Clin. 2010. 26:21–40.

2. Oh YT. Radiation induced lung damage: mechanisms and clinical implications. J Lung Cancer. 2008. 7:9–18.

3. Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995. 31:361–369.

4. Hassaballa HA, Cohen ES, Khan AJ, Ali A, Bonomi P, Rubin DB. Positron emission tomography demonstrates radiation-induced changes to nonirradiated lungs in lung cancer patients treated with radiation and chemotherapy. Chest. 2005. 128:1448–1452.

5. Lo MK, Iupati D, Quddus Z, Carter J, Chander S. A case of sporadic radiation pneumonitis. Am J Clin Oncol. 2006. 29:643–644.

6. Park KJ, Chung JY, Chun MS, Suh JH. Radiation-induced lung disease and the impact of radiation methods on imaging features. Radiographics. 2000. 20:83–98.

7. Gross NJ. Radiation pneumonitis in mice. Some effects of corticosteroids on mortality and pulmonary physiology. J Clin Invest. 1980. 66:504–510.

8. Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005. 201:925–935.

9. Martín C, Romero S, Sánchez-Payá J, Massuti B, Arriero JM, Hernández L. Bilateral lymphocytic alveolitis: a common reaction after unilateral thoracic irradiation. Eur Respir J. 1999. 13:727–732.

10. Kim TH, Cho KH, Pyo HR, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005. 235:208–215.

11. Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000. 48:89–94.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download