Abstract
Lung cancer is a deadly disease that is difficult to diagnose and even more difficult to treat effectively. Many pathways are known to affect tumor growth, and targeting these pathways provides the cornerstone by which cancer is treated. Somatostatin receptors (SSTR) are a family of G protein coupled receptors that signal to alter hormonal secretion, increase apoptosis, and decrease cellular proliferation. These receptors are expressed in many normal and malignant cells, including both small cell and non-small cell lung cancer. Synthetic analogs of SSTRs are commercially available, but their effects in lung cancer are still largely uncertain. Signaling pathway studies have shown that SSTRs signal through phosphotyrosine phosphatases to induce apoptosis as well as to decrease cell proliferation. Radiolabeled SSTR2 analogs are utilized for radiographic imaging of tumors, which, when combined with positron emission tomography-computed tomography (PET-CT) may improve detection of lung cancer. These radiolabeled SSTR2 analogs also hold promise for targeted chemotherapy as well as radiotherapy. In this review, we summarize what is known about SSTRs and focus our discussion on the knowledge as it relates to lung cancer biology, as well as discuss current and future uses of these receptors for imaging and therapy of lung cancer. (J Lung Cancer 2011;10(2):69 ? 76)
Go to : 
REFERENCES
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010; 10:760–774.

3. Reubi JC, Schaer JC, Markwalder R, Waser B, Horisberger U, Laissue J. Distribution of somatostatin receptors in normal and neoplastic human tissues: recent advances and potential relevance. Yale J Biol Med. 1997; 70:471–479.
4. Hejna M, Schmidinger M, Raderer M. The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Ann Oncol. 2002; 13:653–668.

5. Lahlou H, Saint-Laurent N, Estè ve JP, et al. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J Biol Chem. 2003; 278:39356–39371.

6. O'Byrne KJ, Schally AV, Thomas A, Carney DN, Steward WP. Somatostatin, its receptors and analogs, in lung cancer. Chemotherapy. 2001; 47(Suppl 2):78–108.
7. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003; 2:999–1017.

8. Vale W, Rivier J, Ling N, Brown M. Biologic and immu-nologic activities and applications of somatostatin analogs. Metabolism. 1978; 27(9 Suppl 1):1391–1401.

9. Patel YC, Wheatley T. In vivo and in vitro plasma dis-appearance and metabolism of somatostatin-28 and soma-tostatin-14 in the rat. Endocrinology. 1983; 112:220–225.
10. Didden P, Penning C, Masclee AA. Octreotide therapy in dumping syndrome: Analysis of long-term results. Aliment Pharmacol Ther. 2006; 24:1367–1375.

11. Thabut D, Bernard-Chabert B. Management of acute bleeding from portal hypertension. Best Pract Res Clin Gastroenterol. 2007; 21:19–29.

12. Chan JA, Kulke MH. New treatment options for patients with advanced neuroendocrine tumors. Curr Treat Options Oncol. 2011; 12:136–148.

13. Florio T. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol. 2008; 286:40–48.

14. Srikant CB. Cell cycle dependent induction of apoptosis by somatostatin analog SMS 201-995 in AtT-20 mouse pituitary cells. Biochem Biophys Res Commun. 1995; 209:400–406.

15. Liu D, Martino G, Thangaraju M, et al. Caspase-8-mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced apoptosis. J Biol Chem. 2000; 275:9244–9250.

16. Teijeiro R, Rios R, Costoya JA, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mecha-nism that is independent from induction of p53. Cell Physiol Biochem. 2002; 12:31–38.

17. Reubi JC, Waser B, Cescato R, Gloor B, Stettler C, Christ E. Internalized somatostatin receptor subtype 2 in neuroendocrine tumors of octreotide-treated patients. J Clin Endocrinol Metab. 2010; 95:2343–2350.

18. Papotti M, Croce S, Bellò M, et al. Expression of somatostatin receptor types 2, 3 and 5 in biopsies and surgical specimens of human lung tumours. Correlation with preoperative octreotide scintigraphy. Virchows Arch. 2001; 439:787–797.
19. O'Byrne KJ, Halmos G, Pinski J, et al. Somatostatin receptor expression in lung cancer. Eur J Cancer. 1994; 30A:1682–1687.
20. Taylor JE, Theveniau MA, Bashirzadeh R, Reisine T, Eden PA. Detection of somatostatin receptor subtype 2 (SSTR2) in established tumors and tumor cell lines: evidence for SSTR2 heterogeneity. Peptides. 1994; 15:1229–1236.

21. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010; 134:1628–1638.

22. Herlin G, Kö lbeck KG, Menzel PL, et al. Quantitative assessment of 99mTc-depreotide uptake in patients with non-small- cell lung cancer: immunohistochemical correlations. Acta Radiol. 2009; 50:902–908.
23. Taylor JE, Bogden AE, Moreau JP, Coy DH. In vitro and in vivo inhibition of human small cell lung carcinoma (NCI-H69) growth by a somatostatin analogue. Biochem Biophys Res Commun. 1988; 153:81–86.
24. Macaulay VM, Smith IE, Everard MJ, Teale JD, Reubi JC, Millar JL. Experimental and clinical studies with somatostatin analogue octreotide in small cell lung cancer. Br J Cancer. 1991; 64:451–456.

25. Cotto C, Quoix E, Thomas F, Henane S, Trillet-Lenoir V. Phase I study of the somatostatin analogue somatuline in refractory small-cell lung carcinoma. Ann Oncol. 1994; 5:290–291.

26. Ono K, Suzuki T, Miki Y, et al. Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res. 2007; 27:2231–2239.
27. Oddstig J, Bernhardt P, Nilsson O, Ahlman H, Forssell- Aronsson E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl Med Biol. 2006; 33:841–846.
28. Papotti M, Croce S, Macrì L, et al. Correlative immunohistochemical and reverse transcriptase polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol. 2000; 9:47–57.

29. Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinic-opathologic and immunohistochemical study of 218 ‘clinically aggressive' cases. Ann Oncol. 2010; 21:548–555.

30. Muscarella LA, D'Alessandro V, la Torre A, et al. Gene expression of somatostatin receptor subtypes SSTR2a, SSTR3 and SSTR5 in peripheral blood of neuroendocrine lung cancer affected patients. Cell Oncol (Dordr). 2011; 34:435–441.

31. Reubi JC, Schaer JC, Laissue JA, Waser B. Somatostatin receptors and their subtypes in human tumors and in peritumo-ral vessels. Metabolism. 1996; 45(8 Suppl 1):39–41.

32. Blum JE, Handmaker H, Rinne NA. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. Chest. 1999; 115:224–232.

33. Menda Y, Kahn D. Somatostatin receptor imaging of non- small cell lung cancer with 99mTc depreotide. Semin Nucl Med. 2002; 32:92–96.
34. Blum J, Handmaker H, Lister-James J, Rinne N. A multicenter trial with a somatostatin analog (99m)Tc depreotide in the evaluation of solitary pulmonary nodules. Chest. 2000; 117:1232–1238.

35. Mena E, Camacho V, Estorch M, Fuertes J, Flotats A, Carrió I. 99mTc-depreotide scintigraphy of bone lesions in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2004; 31:1399–1404.

36. Kahn D, Menda Y, Kernstine K, et al. The utility of 99mTc depreotide compared with F-18 fluorodeoxyglucose positron emission tomography and surgical staging in patients with suspected non-small cell lung cancer. Chest. 2004; 125:494–501.
37. Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using (68)Ga-DOTATOC PET and comparison with (18)F-FDG PET. Eur J Nucl Med Mol Imaging. 2006; 33:823–830.
38. Ambrosini V, Tomassetti P, Castellucci P, et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2008; 35:1431–1438.

39. Miederer M, Seidl S, Buck A, et al. Correlation of immuno-histopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009; 36:48–52.

40. van Essen M, Krenning EP, Kooij PP, et al. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with para-ganglioma, meningioma, small cell lung carcinoma, and mela-noma. J Nucl Med. 2006; 47:1599–1606.
41. van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009; 5:382–393.

42. Pless M, Waldherr C, Maecke H, Buitrago C, Herrmann R, Mueller-Brand J. Targeted radiotherapy for small cell lung cancer using 90Yttrium-DOTATOC, an Yttrium-labelled somatostatin analogue: a pilot trial. Lung Cancer. 2004; 45:365–371.

43. Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002; 32:148–155.
44. National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.

Go to : 
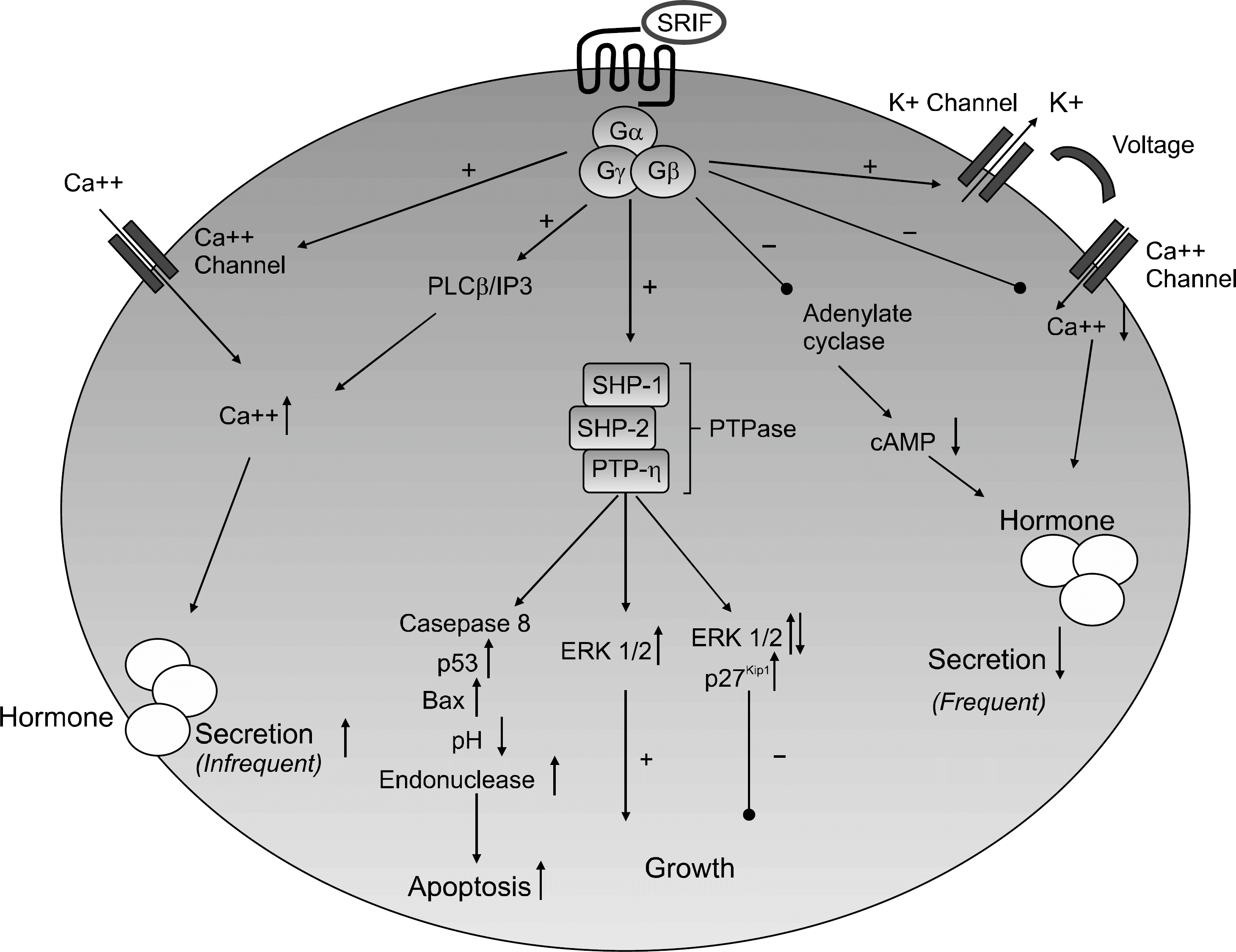 | Fig. 1.Somatostatin receptor sig-naling cascade. SSTR activation decreases hormonal secretion in most cells by decreasing cyclic AMP and by altering voltage gat-ed potassium and calcium cha-nnels. In a minority of cells (pan-creatic B-cells), hormonal secretion is increased. SSTR signals through PTPases to alter ERK1/2 phosphorylation to impair p27 kip1 degradation which leads to cell growth arrest. SSTR also signals through PTPases to induce apoptosis. Rarely SSTR2 induces proliferation. ERK: extracellular signal- regulated kinase, Gα, Gβ, Gγ: G- protein subunit, PLC: phospholi-apse C, cAMP: cyclic adenosine monophosphate, IP3: inositol tri-phosphate, PTPase: phosphotyrosine phosphatase, SRIF: somatostatin, SHP: Src homology phosphatase, PTP-η: phosphotyrosine phosphatase η. |
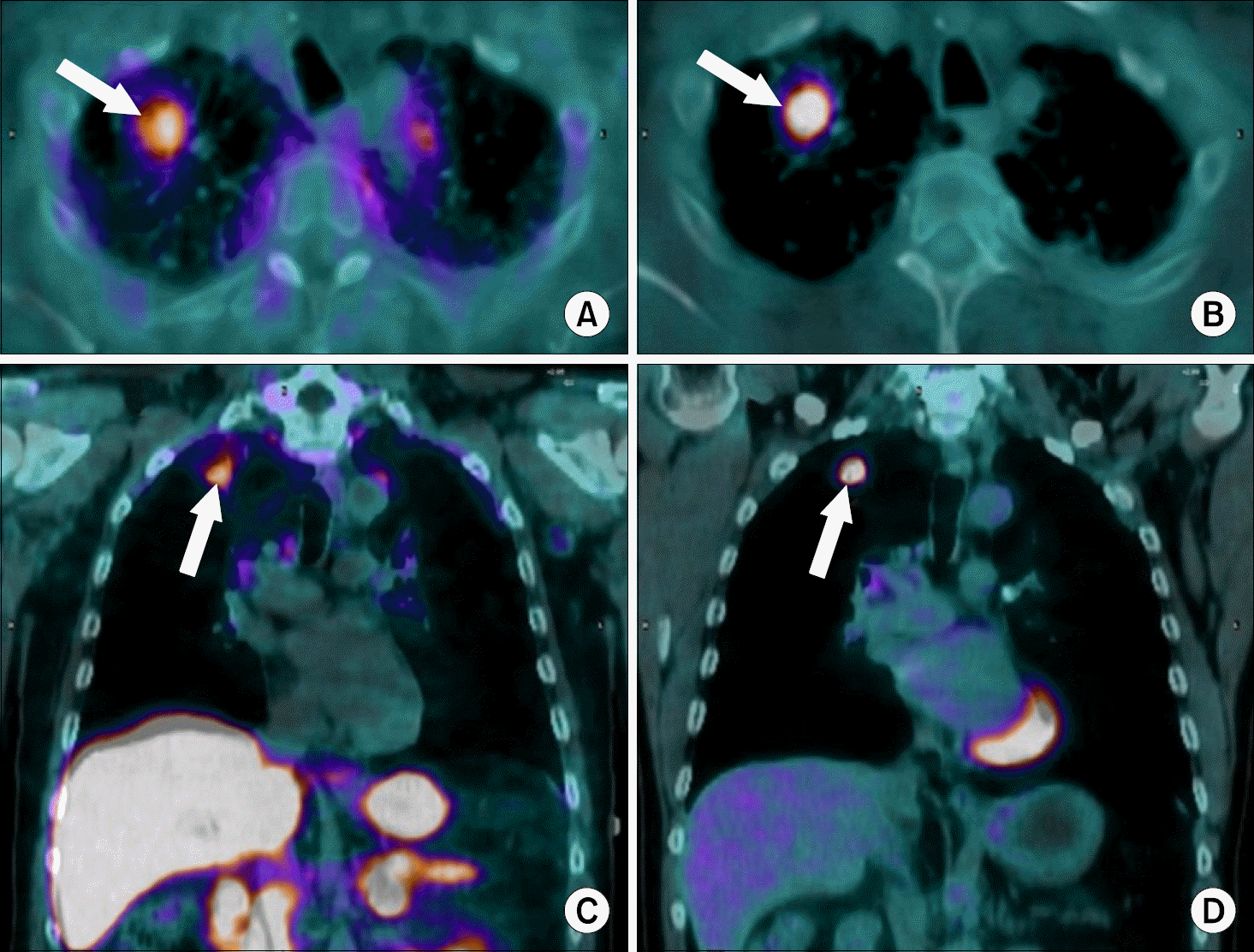 | Fig. 2.Use of a new synthetic somatostatin analog in lung cancer: Axial (A, B) and coronal (C, D) fused PET/CT images in a patient with newly diagnosed, un-treated adenocarcinoma of the right upper lobe (arrows). The tumor is easily seen with both the somatostatin analog (68 Ga-DOTATATE; A, C) and with conventional PET imaging (18 F-FDG: B, D) with com-parable uptake (each had an SUV value of 2.7). The combination of both imaging agents may help reduce false positive exams with 18 F-FDG PET/CT imaging alone, decreasing unnecessary biopsies or thoracotomies. Images by the authors. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download