Abstract
30-year-old male was admitted with general weakness and drowsy mental status. He had eaten only 3-4 spoons of brown rice and fresh vegetable without salt for 3 months to treat his tic disorder, and he had been in bed-ridden state. He has had weight loss of 14 kg in the last 3 months. We report a patient with orthorexia nervosa who developed hyponatremia, metabolic acidosis, subcutaneous emphysema, mediastinal emphysema, pneumothorax, and pancytopenia and we will review the literature. Also, we mention to prevent refeeding syndrome, and to start and maintain feeding in malnourished patients.
Go to : 
Orthorexia nervosa is a new eating disorder described by Dr. Steven Bratman in 2007. Orthorexia nervosa is an obsession for healthy food which may lead to strict diets, sometimes with a shortage of essential nutrients. Patients with orthorexia nervosa are worried about quality of food, in contrast patients with anorexia and bulimia are worried about its quantity1).
Patients with malnutrition due to anorexia nervosa were reported with severe hyponatremia2), hypokalemia with renal potassium wasting, and metabolic acidosis with a normal plasma anion gap3), subcutaneous emphysema, mediastinal emphysema, pneumothorax4), and pancytopenia5).
Refeeding syndrome is defined as the potentially fatal shifts in fluids and electrolytes that may occur in malnourished patients by aggressive reinstitution of nutritional support6). The hallmark biochemical feature of refeeding syndrome is hypophosphataemia. Refeeding syndrome characterizes abnormal sodium and fluid balance, changes in glucose, protein, and fat metabolism, thiamine deficiency, hypokalaemia, and hypomagnesaemia along with alterations in neurologic, cardiac, hematologic, neuromuscular, and pulmonary function6).
We report a patient with orthorexia nervosa who developed hyponatremia, metabolic acidosis, subcutaneous emphysema, mediastinal emphysema, pneumothorax, and pancytopenia and we review the literature. Also, we discuss preventive measures in refeeding syndrome, to identify high risk patients and introduce American and European guidelines.
Go to : 
Case : A 30-year-old male was admitted to our hospital with general weakness and drowsy mental status. He had eaten only 3-4 spoons of brown rice and fresh vegetable without salt for 3 months to treat his tic disorder, and he had been in bed-ridden state. He had taken propolis and enema by himself, and his past medical history was unremarkable except tic disorder since high school. His blood pressure was 100/70 mm Hg, pulse rate 72/min, and respiratory rate 18/min, body temperature 36℃. He had lost 14 kg in the past 3 months. Physical examination presented cracking over his skin around his neck and both shoulders.
Serum laboratory results were as follows; hemoglobin 16.2 g/dL, white blood cell (WBC) 4.9 × 103/µL, platelet 201 × 103/µL, total protein 5.6 g/dL, albumin 3.4 g/dL, aspartate aminotransferase (AST) 114 IU/L, alanine aminotransferase (ALT) 101 IU/L, alkaline phosphatase (ALP) 57 IU/L, γ-glutamyl transferase (GGT) 13 IU/L, bilirubin (total/direct) 3.22/0.8 mg/dL, glucose 166 mg/dL, amylase 98 IU/L, blood urea nitrogen (BUN) 33.6 mg/dL, creatinine 0.41 mg/dL, total CO2 20.2 mEq/L, sodium 101 mEq/L, potassium 4.0 mEq/L, chloride 68 mEq/L, calcium 6.8 mg/dL, inorganic phosphorus 2.7 mg/dL, magnesium 1.5 mEq/L, uric acid 1.0 mg/dL, C-reactive protein (CRP) 1.87 mg/dL, osmolality 213 mOsm/kg H2O, creatine phosphokinase (CPK) 1,472 IU/L (29-145), myoglobin 1,278 ng/mL, lactate dehydrogenase (LDH) 1,472 IU/L (0-500), total cholesterol 231 mg/dL, triglyceride 97 mg/dL, high density lipoprotein (HDL)-cholesterol 127 mg/dL, low density lipoprotein (LDL)-cholesterol 83 mg/dL, prealbumin 10.7 mg/dL, hemoglobin A1c 5.4%, thyroid stimulating hormone (TSH) 1.26 uIU/mL, free T4 0.75 ng/dL, and international normalized ratio (INR) 1.61. Urinalysis revealed a specific gravity of 1.012, pH 7.0, trace protein, glucose 2+, occult blood 3+, myoglobin 2,687 ng/m, urine sodium 62 mEq/L, potassium 34.4 mEq/L, chloride 43.1 mEq/L, and osmolality 484 mOsm/kg H2O.
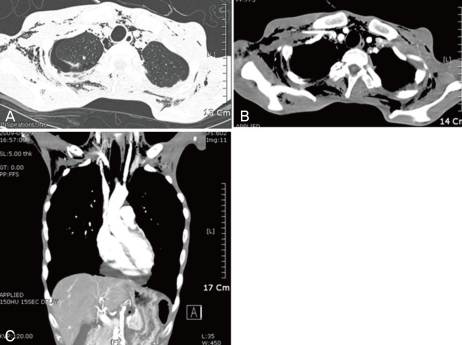
Chest AP X-ray showed mediastinal emphysema and extensive soft tissue emphysema in his neck and chest wall. Abdominal ultrasonography revealed a small amount of ascites and small amount of fluid collection in his bilateral perinephric space. Chest CT showed extensive soft tissue emphysema in his neck and chest wall, extensive mediastinal emphysema, small right pneumothorax, and pericardial fluid (Fig. 1). To rule out esophageal perforation esophagography with gastrographin was performed and it showed no evidence of contrast leakage from his esophagus. The diagnosis of orthorexia nervosa with hyponatremia, rhabdomyolysis, pneumomediastinum, pneumothorax, and soft tissue emphysema was made. We started on intravenous fluid supplementation based on treatment of hyponatremia and refeeding syndrome, and oral feeding. He gradually improved, but his total protein and albumin decreased on the 5th hospital day to 4.4 g/dL and 2.5 g/dL, respectively. On the 7th hospital day, his platelet and WBC levels decreased to 27 × 103/µL and 1.2 × 103/µL, respectively. On the 12th hospital day his hemoglobin level decreased to 7.2 g/dL from his hospital 2nd day. Peripheral blood smear showed normochromic normocytic anemia with anisopoikilocytosis 2+, neutropenia, lymphocytopenia, relative monocytosis 14%, and thrombocytopenia. His iron level was 88 µg/dL, total iron binding capacity (TIBC) 168 µg/dL, ferritin 908.6 ng/mL, vitamin B12 988 pg/mL, folate 2.8 ng/mL, and his Coomb's test, plasma hemoglobin, haptoglobin, and serology were within normal limits. We transfused 6 units of platelet concentrate by his hospital 7th day and granulocyte colony-stimulating factor (G-CSF) 150 µg due to absolute neutrophil count (ANC) 560 and methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia by his hospital 16th day. By his hospital 8th day, serum laboratory results were as follows; sodium 138 mEq/L, potassium 3.7 mEq/L, chloride 99 mEq/L, calcium 8.1 mg/dL, inorganic phosphorus 1.9 mg/dL, magnesium 1.4 mEq/L, total CO2 32.5 mEq/L, osmolality 271 mOsm/kg H2O, CPK 270 IU/L, myoglobin 57.8 ng/mL, LDH 1,011 IU/L, and total cholesterol 140 mg/dL. By hospital 18th day, his AST level was 25 IU/L, ALT 59 IU/L, ALP 89 IU/L, bilirubin (total/direct) 0.35/0.12 mg/dL, and by hospital 28th day, his LDH was 514 IU/L. Until his hospital 20th day, his platelet level steadily increased to 1,475 × 103/µL, and gradually decreased to 382 × 103/µL by the hospital 37th day. By his hospital 37th day, his hemoglobin increased to 12.1 g/dL and WBC also stabilized to 3.4 × 103/µL (neutrophil 48%).
He was discharged by his hospital 38th day in an improving state, and followed up at our rehabilitation department for physical therapy and psychological department for supportive therapy.
Go to : 
Orthorexia nervosa is similar to anorexia nervosa. Patients with anorexia nervosa were reported as severe hyponatremia due to 1) inadequate fluid and sodium intake from anorectic behaviors and dehydration from self-induced vomiting, laxative or diuretic abuse, 2) excessive consumption of water due to hunger, 3) Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) by selective serotonin reuptake inhibitors (SSRIs) and phenothiazines, and 4) impaired water diuresis due to low protein intake2,7).
Our patient's hyponatremia can be explained by impaired water diuresis and resetting osmostat. Especially, the physiology of water diuresis is impaired at multiple levels in patients with anorexia nervosa2). Low blood urea nitrogen from low protein intake decreases the glomerular filtration pressure and medullary hypertonicity. Sodium reabsorption is impaired in the proximal tubule due to solute delivery by low glomerular filtration2), and in the thin ascending loop of Henle due to diminished medullary hypertonicity2). Furthermore, the chronic hyponatremic state can often persist due to a "resetting of the osmostat" in response to a low serum osmolality2). In patients with anorexia nervosa diminished water diuresis is directly related to the severity of malnutrition2). Patients with anorexia nervosa may be complicated with central pontine myelinolysis in the absence of obvious neurologic deficits by rapid correction8). To avoid brain damage, sodium should be substituted at a speed not exceeding 0.5 mEq/L/hour8).
Our patient showed features of rhabdomyolysis. The pathogenesis of rhabdomyolysis due to hyponatremia is thought to be a result of hypo-osmolality of the extracellular fluid, leading to muscle cell swelling9). After the cellular swelling normalizes, as a result of extrusion of intracellular potassium, the potassium-depleted muscle cells fail to release potassium, and blood flow becomes insufficient9). The cellular transmembrane potential is decreased, leading to release of creatine kinase and myoglobin9).
Our patient showed normal anion gap acidosis. The HCO3- buffer system selectively removes H+. Two factors in this patient could contribute to poor buffering of H+; 1) A contracted extracellular fluid (ECF) volume can lead to a lower blood flow rate and to a high capillary and intracellular PCO2, 2) Although most intracellular fluid (ICF) buffering occurs in skeletal muscle, our patient was emaciated, and thus, his buffer capacity was markedly reduced3). This probably led to a more severe degree of acidemia for a given deficit of NaHCO3. It was advisable to treat his metabolic acidosis with isotonic saline to remove the limitation of a high tissue PCO2 on his ICF HCO3- buffer system3).
Our patient revealed extensive soft tissue emphysema in his neck and chest wall, extensive mediastinal emphysema, and a small amount of right pneumothorax. Comorbidity of anorexia nervosa with pneuumomediastinum or pneumothorax rarely has been described in the literature4). Malnutrition of anorexia nervosa can lead to a heightened proneness to emphysema and rupture of pulmonary tissue by empty vomiting and oral manipulation leading to injury of the oral cavity4). Our patient had spontaneous emphysema and pneumothorax, and we conservatively treated the patient with O2 therapy.
During his hospital course, our patient developed pancytopenia, and improved with one dose of G-CSF, and 6 units of platelet transfusion. Hematologic features of anorexia nervosa are characterized by pancytopenia, bone marrow change, marrow cell necrosis, and a relative increase in lymphocytes and plasma cells. Fatal cases have been reported5,7). Carbohydrate deficiency causes bone marrow hypoplasia with accumulation of gelatinous acid mucopolysaccharide, and protein deficiency causes hypoplasia without gelatinous transformation5). Anemia in anorexia nervosa is not caused by simple iron deficiency, but due to lipoprotein abnormality5). Leukocytopenia and a decreased rate of in vitro killing of bacteria, and impaired cell-mediated immunity, induce a widespread infection5). Thrombocytopenia is considered to be due to hypoplastic bone marrow5).
Our patient had increased AST, ALT, and total bilirubin, and improved spontenously. Miller et al. reported elevation of ALT, 12.2% in 214 women with anorexia nervosa7).
Refeeding syndrome is a fatal complication during artificial feeding-parenteral or enteral- in malnourished patients. Potential complications of refeeding syndrome include fatal cardiac arrhythmia, systolic heart failure, respiratory insufficiency, and h ematologic derangements6).
During the period of prolonged starvation, several intracellular minerals become severely depleted10-12). However, serum concentrations of these minerals including phosphate may remain normal. This is because these minerals are mainly in the intracellular compartment, which contracts during starvation. In addition, there is a reduction in renal excretion10-12).
During refeeding, glycemia leads to increased insulin and decreased secretion of glucagon. Insulin stimulates glycogen, fat, and protein synthesis. This process requires minerals such as phosphate and magnesium and cofactors such as thiamine. Insulin stimulates the absorption of potassium into the cells through the sodium-potassium ATPase symporter, which also transports glucose into the cells. Magnesium and phosphate are also taken up into the cells. Water follows by osmosis. These processes result in a decrease in the serum levels of phosphate, potassium, and magnesium; all of which are already depleted6,10-12).
The clinical features of the refeeding syndrome occur as a result of the functional deficits of these electrolytes and the rapid change in basal metabolic rate. Although all vitamin deficiencies may occur at variable rates with inadequate intake, thiamine is of most importance in complications of refeeding. Thiamine is an essential coenzyme in carbohydrate metabolism. Thiamine deficiency results in Wernicke's encephalopathy (ocular abnormalities, ataxia, confusional state, hypothermia, coma) or Korsakoff's syndrome (retrograde and anterograde amnesia, confabulation)10-12).
Criteria from the guidelines of the National Institute for Health and Clinical Excellence (NICE) for identifying patients at high risk of refeeding problems are one or more of the following: body mass index < 16 kg/m2, unintentional weight loss > 15% in the past 3 to 6 months, little or no nutritional intake for > 10 days, and low levels of potassium, phosphate, or magnesium before feeding10). Patients at high risk of refeeding problems are two or more of the following: body mass index < 18.5 kg/m2, unintentional weight loss > 10% in the past 3 to 6 months, little or no nutritional intake for > 5 days, and history of alcohol misuse or drugs, including insulin, chemotherapy, antacids, or diuretics10).
Before feeding starts, NICE recommends administering thiamine 200-300 mg daily orally, vitamin B high potency 1-2 tablets 3 times daily or full dose intravenous vitamin B, and multivitamin or trace element supplement once daily10). NICE recommends starting feeding at 0.0418 MJ/kg/day (10 kcal/kg/day, no more than 50% of energy requirements, in patients with body mass index < 14 or a negligible intake for 2 weeks or more NICE recommend to start feeding 0.021 MJ/kg/24 hrs), and slowly increasing feeding over 4-7 days10). NICE recommends rehydrating carefully and supplementing and/or correcting levels of potassium (give 2-4 mEq/kg/day), phosphate (0.3-0.6 mmol/kg/day), calcium, and magnesium (0.2 mmol/kg/day intravenously or 0.4 mmol/kg/day orally)10). NICE recommends monitoring potassium, phosphate, calcium, and magnesium for the first 2 weeks, and amending treatment as needed10). European guidelines recommend restricting fluid to maintain renal function, replacing deficits or losses, and avoiding weight gain, that is zero balance. Patients usually need 20-30 mL/kg/day during Days 1-311). European guidelines recommend restricting sodium to < 1 mEq/kg/day. If edema develops, further restriction during Days 1-3 should be done11). European guidelines recommend that Iron should not be supplemented in the first week during Days 1-311). European guidelines recommend that energy 15-20 kcal/kg/day, phosphate 30-50 mmol IV over 12 hours in phosphorus < 0.5 mmol/L, MgSO4 24 mmol IV over 12 hours in Mg2+ < 0.5 mmol/L, KCl 20-40 mEq IV over 4 hours in potassium < 3.5 mEq/L, and fluid 25-30 mL/kg/day during Days 4-6 should be done11). European guidelines recommend that energy 20-30 kcal/kg/day, iron supplementation from day 7, and fluid 30 mL/kg/day during Days 7-10 should be done11).
In conclusion, orthorexia nervosa and anorexia nervosa have variable manifestations including fatal complications. Before feeding, we should evaluate the comorbidity, and consider the possibility of refeeding syndrome to eliminate fatal complications, and start feeding and behavior therapy.
Go to : 
References
1. Kummer A, Dias FM, Teixeira AL. On the concept of orthorexia nervosa. Scand J Med Sci Sports. 2008; 18:395–396. author reply 397. PMID: 18435688.

2. Bahia A, Chu ES, Mehler PS. Polydipsia and hyponatremia in a woman with anorexia nervosa. Int J Eat Disord. 2011; 44:186–188. PMID: 20127934.

3. Luthra M, Davids MR, Shafiee MA, Halperin ML. Anorexia nervosa and chronic renal insufficiency: a prescription for disaster. QJM. 2004; 97:167–178. PMID: 14976274.

4. Danzer G, Mulzer J, Weber G, Lembke A, Kocalevent R, Klapp BF. Advanced anorexia nervosa, associated with pneumomediastinum, pneumothorax, and soft-tissue emphysema without esophageal lesion. Int J Eat Disord. 2005; 38:281–284. PMID: 16211634.

5. Fukudo S, Tanaka A, Muranaka M, et al. Case report: reversal of severe leukopenia by granulocyte colony-stimulating factor in anorexia nervosa. Am J Med Sci. 1993; 305:314–317. PMID: 7683451.

6. Marinella MA. Refeeding syndrome: an important aspect of supportive oncology. J Support Oncol. 2009; 7:11–16. PMID: 19278172.
7. Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005; 165:561–566. PMID: 15767533.

8. Amann B, Schafer M, Sterr A, Arnold S, Grunze H. Central pontine myelinolysis in a patient with anorexia nervosa. Int J Eat Disord. 2001; 30:462–466. PMID: 11746309.

9. Trimarchi H, Gonzalez J, Olivero J. Hyponatremia-associated rhabdomyolysis. Nephron. 1999; 82:274–277. PMID: 10396001.

10. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008; 336:1495–1498. PMID: 18583681.

11. Stanga Z, Brunner A, Leuenberger M, et al. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008; 62:687–694. PMID: 17700652.

12. Azumagawa K, Kambara Y, Kawamura N, et al. Anorexia nervosa and refeeding syndrome. A case report. ScientificWorldJournal. 2007; 7:400–403. PMID: 17370025.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download