Abstract
This is a case of a sudden cardio-pulmonary arrest in a 29 year-old female, which occurred immediately after a large bolus infusion of propofol (100 mg) intravenously during dilatation and curettage. The arrest suddenly occurred, and the patient was eventually transferred to our emergency room (ER) on cardiopulmonary resuscitation. At that time, severe hyperkalemia up to 9.1 mEq/L and ventricular fibrillation were noted. Resuscitation in ER worked successfully with conversion of electrocardiograph to sinus rhythm, but this patient expired unfortunately. On view of this acute event immediately after the bolus injection of propofol accompanied without other identified causes, severe hyperkalemia induced by propofol was strongly assumed to be the cause of death. To our understanding with the literature survey, propofol as a cause of hyperkalemia has not been well described yet. Through this case, the relationship as a cause and an effect between propofol and hyperkalemia is suggested.
Go to : 
Propofol is widely used in anesthesia at intensive care units and out-patients-unit procedures due to its short term sedation effects and safe pharmacodynamics. However, propofol infusion syndrome (PRIS) was suspected in pediatric patients in the 1990s1) and then Bray et al.2) introduced the term first in 1998. After that, PRIS was also observed in adult patients3). PRIS is a rare, but frequently fatal complication of prolonged propofol infusion at high doses. This syndrome is characterized by metabolic acidosis, arrhythmias, myocardial failure, rhabdomyolysis, hyperkalemia, renal failure, fatty degeneration of liver, and then eventually causes death in some cases4,5). Currently, PRIS is assumed to be related with disruption of fatty acid oxidation and failure of the mitochondrial respiratory chain3,6,7).
The development of hyperkalemia has been reported in patients after propofol infusion anaesthesia6,8-10), suggesting that hyperkalemia could be associated with propofol infusion. However, there is no case report of sudden cardiopulmonary arrest immediately after propofol infusion accompanied by severe hyperkalemia. In addition, the relationship between propofol and hyperkalemia is not clarified yet. Hereby, we report a case in which propofol was assumed to cause severe hyperkalemia leading to death, and propose the relationship between propofol itself and the development of hyperkalemia.
Go to : 
A 29-year-old female was transferred to our emergency room (ER), in cardiopulmonary arrest state which occurred during dilatation and curettage at a local obstetric unit. The dilatation and curettage were performed due to intrauterine fetal death at 24 weeks of gestation. During the procedure, bolus injection of propofol (100 mg in 10 mL) was used for sleep induction. Electrocardiography (EKG) showed sinus rhythm before the propofol was injected. Approximately 10 seconds after its injection, cyanosis appeared on the lips with oxygen saturation falling to 40%. Consequently, the arrest occurred, and intubation with cardiopulmonary resuscitation (CPR) was performed. Then, the patient was transferred to the ER, at Hanyang University Guri Hospital, with continuation of CPR. Forty minutes passed before her arrival to the ER, but asystole was still present in the ER. Crackle sound was heard from both lungs and pulmonary edema was noted on the chest X-ray.
The hematological study showed white cells at 21 × 103/µL, hemoglobin 10.4 g/dL, hematocrit 31.7%, and platelets 238 × 103/µL. The serum electrolytes showed a sodium level of 128 mEq, potassium 9.1 mEq/L, and chloride 97 mEq/L with blood chemistry of creatinine at 0.6 mg/dL, urea nitrogen 11 mg/dl, bilirubin 0.6 mg/dL, aspartate aminotrasferase 1,088 U/L, alanine aminotransferase 548 U/L, total protein 6.2 g/dL, albumin 2.8 g/dL, alkaline phosphatase 966 U/L, lactate dehydrogenase 9,135 U/L, creatine kinase 1,146 U/L, calcium 9.7 mg/dL, phosphorus 13.1 mg/dL, creatine kinase isoenzymes 125 ng/mL, myoglobulin 1,451 ng/mL, troponin-I 18.38 ng/mL, glucose 340 mg/dL, and osmolality 305 mOsm/kg. The blood gas revealed pH of 6.99, PCO2 52.7 mmHg, PO2 90.7 mmHg, bicarbonate 13.0 mEq/L, base-excess -17.6, and O2 saturation 90%.
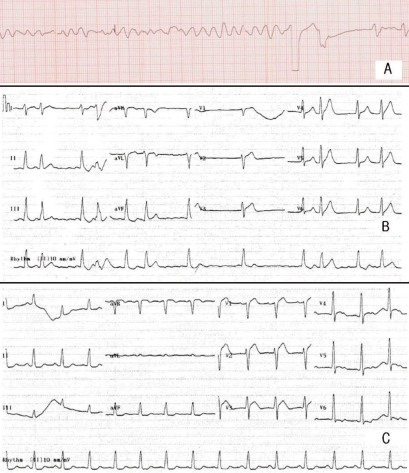
To control hyperkalemia, 10% calcium gluconate and 10 units insulin mixed with 50 mL of 50% glucose fluid were infused, and after 10 minutes, the patient seemed to be resuscitated. However, 6 minutes later, ventricular fibrillation occurred and defibrillation was conducted immediately. Before the conversion to sinus rhythm was accomplished consequently, symmetric tall T waves with atrial fibrillation followed temporarily (Fig. 1). Then, blood pressure and pulse rate were maintained at 109/47 mmHg and 68 per minute, respectively, with inotropics. Stuporous mental status and complete anuria was noted despite intravenous furosemide injection up to 160 mg in total but the repeat levels of serum creatinine and potassium in the ER were 0.9 mg/dL and 7.7 mEq/L, respectively. Her family members refused further management in our intensive care unit. She was moved to a local medical unit near her home and expired there within 1 day.
Go to : 
A healthy 29-year-old female in pregnancy developed cardiopulmonary arrest while receiving dilatation and curettage, right after propofol was injected. In spite of the CPR, resuscitation wasn't successful until the management of hyperkalemia was initiated. In this case, there could be much debate on the causes of the arrest, such as respiratory failure with airway problem, amniotic fluid embolism, anaphylaxis, and PRIS. As common accidents of other sedatives, airway problem would be suspected first, but the interval between injection and arrest was too short (about 10 seconds) to explain that hypoxia alone caused the cardiopulmonary arrest. As intubation was performed immediately, even if it was due to the respiratory failure, the patient should have been resuscitated after oxygen supply. Amniotic fluid embolism could occur during dilatation and curettage. But as described in the case reported by Ray et al.11), blood potassium level did not rise typically and usually recovered after CPR. In addition, typical features of disseminated intravascular coagulation (DIC) including thrombocytopenia weren't seen in this patient. Also, among the usual cases of anaphylaxis, they did not develop severe hyperkalemia and ventricular fibrillation right after its development. Furthermore, Castillo et al. described that propofol under 2 mg/kg should be safe in local anesthesia of delivery12).
PRIS is one of the well known side effects of propofol as mentioned above. The present case is somewhat different from previously reported PRIS cases. PRIS usually occurs when propofol is used in doses of more than 4 mg/kg/hr for more than 48 hours6). On the contrary, in this case, cardiopulmonary arrest occurred directly after an intravenous bolus injection of propofol. So far, 71 PRIS cases are reported. But none of them occurred right after the injection of propofol. The shortest onset time of PRIS was at least 40 minutes6,9). Among 61 cases of PRIS6) reviewed by Kam and Cardone, hyperkalemia was only noted in 8 cases out of a total of 38 expired ones, but none in survived cases. In a case of PRIS reported by Strikland and Murray, metabolic acidosis and hyperkalemia occurred after continuous infusion of the propofol, but hyperkalemia was aggravated causing death in spite of improvement of metabolic acidosis by bicarbonate infusion13). On the other hand, Vernooy et al.10) reported that 3 of the 7 PRIS patients and 6 of the 60 non-PRIS patients had hyperkalemia, among a total of 67 patients who received long term propofol. These indicate that propofol could develop hyperkalemia regardless of the clinical status either with or without PRIS.
Since there was no evidence of DIC, no significant elevation of muscle enzymes, and within normal range of serum creatinine, it is hard to suggest that hemolysis, muscle cell destruction or acute renal failure induced severe hyperkalemia up to a level of 9.1 mEq/L as in this case. After emergent attempts were conducted for severe hyperkalemia including calcium gluconate, and insulin with glucose, the patient was resuscitated. Notably, ventricular fibrillation had changed to atrial fibrillation with symmetric tall T waves, and then to sinus rhythm with normal T waves after severe hyperkalemia was managed with emergent remedies. This evidence suggests that the immediate cardiopulmonary arrest could have been caused by hyperkalemia, of which cause is related to the propofol.
Succinylcholine has been known to cause hyperkalemia by diffusion of potassium during prolonged muscle depolarization14). In an interesting case reported by Piotrowski et al.15), hyperkalemia and cardiac arrest occurred in a patient immediately after anesthesia using both succinylcholine and propofol. However, no side effect was observed after initial succinylcholine administration in this patient. It could be possible that the cardiac arrest and hyperkalemia were induced by propofol rather than succinylcholine. Another similar case showed hyperkalemia and polymorphic ventricular tachycardia, which occurred during resuscitation on the arrest developed right after injection of propofol and succinylcholine16).
The main mechanism of PRIS could be explained through the disturbance of the mitochondrial fatty acid metabolism. The mechanism of PRIS could be similar to mitochondrial myopathy, in which there are specific defects in the mitochondrial respiratory chain3). A case of hyperkalemia with severe metabolic acidosis in an infant with mitochondrial fatty acid oxidation disorder, suggests that potassium homeostasis could have a relationship with mitochondrial metabolism17). Meanwhile, propofol could develop lactic acidemia through the impairment of free fatty acid utilization and mitochondrial activity in muscles and heart18). In a recent report, compared to another anesthetic agent, propofol has shown significant effects that produce lactic acidosis18). However, as hyperkalemia does not occur in organic metabolic acidosis14), hyperkalemia of propofol would not have any correlations with lactic acidosis.
Despite the lack of clarified mechanisms that explain how propofol could influence potassium homeostasis, there is a possible suggestion of a proven effect of propofol in an animal study. Zhou et al. found that propofol could decrease beta-adrenoceptor responsiveness to isoproterenol in rats19) which may imply that propofol could have effects to block the intracellular potassium shift of beta-agonists. Severe acute hyperphosphatemia, which was probably the result of extracellular shift of phosphate along with potassium without the influence of tumor lysis syndrome or significant rhabdomyolysis, may be another indicator that the cause of hyperkalemia was transcellular movement rather than that of other mechanisms. In fact, transcellular movement of potassium is usually accompanied by the same directional movement of phosphate with insulin or change of pH and even in thyrotoxic periodic paralysis20). Derangement of internal balance of potassium homeostasis, transcellular shift of potassium is strongly suggested as this cause of acute onset hyperkalemia rather than derangement of external balance of potassium in this patient, although urinary biochemical indices such as transtubular potassium gradient (TTKG) were not taken.
In the body, most of the potassium is contained in muscle and liver. PRIS usually associates with rhabdomyolysis, so the occurrence of hyperkalemia could be secondary by rhabdomyolysis. In some reports, however, hyperkalemia occurred without rhabdomyolysis. In the review of Kam and Cardone, 5 out of 11 patients had hyperkalemia without rhabdomyolysis6). In the report of Vernooy et al., 6 out of 60 non-PRIS patients had hyperkalemia10). Moreover, the patient's CPK level was just 1,146 U/L, not so high for significant rhabdomyolysis.
In conclusion, considering all of the clinical clues and a review of the data from the previous reports of humans and animal experiments about propofol, there may be a close relationship between the development of severe hyperkalemia and propofol in this report. However, there still remains lack of evidence that could explain the exact pathophysiology of hyperkalemia following propofol injection, despite various speculation including depressed beta adrenoceptor-agonist effect and transcelluar movement of potassium along with phosphate.
Go to : 
Acknowledgements
The authors thank Dr. Man S. Oh, State University of New York, Health Science Center at Brooklyn, New York, USA, for his crucial comments and critical reading of the manuscript.
Go to : 
References
2. Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998; 8:491–499. PMID: 9836214.

3. Wolf A, Weir P, Segar P, Stone J, Shield J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet. 2001; 357:606–607. PMID: 11558490.

5. Fodale V, La Monaca E. Propofol infusion syndrome: an overview of a perplexing disease. Drug Saf. 2008; 31:293–303. PMID: 18366240.
7. Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003; 29:1417–1425. PMID: 12904852.

8. Ahlen K, Buckley CJ, Goodale DB, Pulsford AH. The 'propofol infusion syndrome': the facts, their interpretation and implications for patient care. Eur J Anaesthesiol. 2006; 23:990–998. PMID: 16938158.

9. Fudickar A, Bein B, Tonner PH. Propofol infusion syndrome in anaesthesia and intensive care medicine. Curr Opin Anaesthesiol. 2006; 19:404–410. PMID: 16829722.

10. Vernooy K, Delhaas T, Cremer OL, et al. Electrocardiographic changes predicting sudden death in propofol-related infusion syndrome. Heart Rhythm. 2006; 3:131–137. PMID: 16443524.

11. Ray BK, Vallejo MC, Creinin MD, et al. Amniotic fluid embolism with second trimester pregnancy termination: a case report. Can J Anaesth. 2004; 51:139–144. PMID: 14766690.

12. Castillo T, Avellanal M, Garcia de Lucas E. Bolus application of remifentanil with propofol for dilatation and curettage. Eur J Anaesthesiol. 2004; 21:408–411. PMID: 15141801.

13. Strickland RA, Murray MJ. Fatal metabolic acidosis in a pediatric patient receiving an infusion of propofol in the intensive care unit: is there a relationship? Crit Care Med. 1995; 23:405–409. PMID: 7867366.
14. Oh MS. Disorders of potassium: Acid-base electrolytes. 2003. New York: Ohco. LLC;p. 146.
15. Bonhomme V, Demoitie J, Schaub I, Hans P. Acid-base status and hemodynamic stability during propofol and sevoflurane-based anesthesia in patients undergoing uncomplicated intracranial surgery. J Neurosurg Anesthesiol. 2009; 21:112–119. PMID: 19295389.

16. Jackson MA, Lodwick R, Hutchinson SG. Hyperkalaemic cardiac arrest successfully treated with peritoneal dialysis. BMJ. 1996; 312:1289–1290. PMID: 8634622.
17. Wasant P, Matsumoto I, Naylor E, Liammongkolkul S. Mitochondrial fatty acid oxidation disorders in Thai infants: a report of 3 cases. J Med Assoc Thai. 2002; 85(Suppl 2):S710–S719. PMID: 12403251.
18. Bonhomme V, Demoitie J, Schaub I, Hans P. Acid-base status and hemodynamic stability during propofol and sevoflurane-based anesthesia in patients undergoing uncomplicated intracranial surgery. J Neurosurg Anesthesiol. 2009; 21:112–119. PMID: 19295389.

19. Zhou W, Fontenot HJ, Wang SN, Kennedy RH. Propofol-induced alterations in myocardial beta-adrenoceptor binding and responsiveness. Anesth Analg. 1999; 89:604–608. PMID: 10475288.
20. Lin YF, Wu CC, Pei D, Chu SJ, Lin SH. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003; 21:339–342. PMID: 12898495.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download