PI3K/Akt signaling
1. Akt phosphorylation induced by the phosphatidylinositol 3-kinase (PI3K) activation
2. Expression of Akt isoforms
Trafficking of membrane protein by Akt signaling
1. Trafficking of membrane protein regulated by PI3K/Akt signaling
2. Akt signaling-associated Rab proteins in membrane protein trafficking
Akt signaling and Akt-associated Rab proteins in AQP2 membrane trafficking
1. Akt signaling for regulation of AQP2 protein expression
2. Akt-dependent signaling pathway for the regulation of AQP2 trafficking
Results
1. Regulation of AQP2 expression in M-1 cells in response to dDAVP treatment
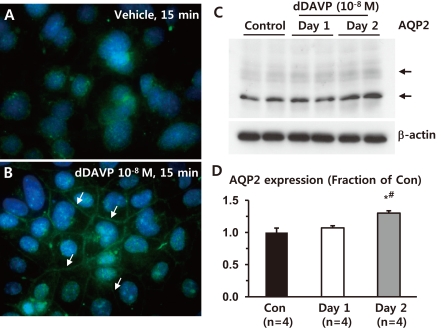 | Fig. 1Regulation of aquaporin-2 (AQP2) in response to dDAVP treatment in mouse cortical collecting duct cells (M-1 cells). A and B) Immunofluorescence microscopy of AQP2. Short-term dDAVP-treatment (10-8 M) for 15 minutes in M-1 cells increased AQP2 trafficking to the plasma membrane (indicated by arrows in B), compared with vehicle-treated M-1 cells (A). C and D) Semiquantitative immunoblotting of AQP2. Immunoblots reacted with anti-AQP2 revealed 29- and 35- to 50-kDa AQP2 bands, representing nonglycosylated and glycosylated forms of AQP2, respectively. AQP2 expression was significantly increased in 2 day-dDAVP-treated M-1 cells, compared with either vehicle-treated or 1 day-dDAVP-treated M-1 cells. n, the number of separate M-1 cell lysates.
*P < 0.05 when compared to the vehicle-treated control group.
†P < 0.05 when compared to the 1 day-dDAVP treated group.
|
2. Decreased Akt1 but unchanged AQP2 expression in M-1 cells with Akt1 knockdown
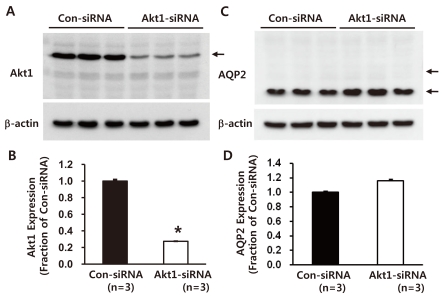 | Fig. 2Semiquantitative immunoblotting of Akt1 and AQP2, aquaporin-2 (AQP2) in mouse cortical collecting duct cells (M-1 cells) with Akt1 knockdown. A and B) Immunoblots reacted with anti-Akt1 revealed 60-kDa Akt1 bands. Akt1-directed small interfering RNA (siRNA) treatment decreased Akt1 expression. C and D) Immunoblots reacted with anti-AQP2 revealed 29- and 35- to 50-kDa AQP2 bands, representing nonglycosylated and glycosylated forms of AQP2, respectively. AQP2 expression was unchanged in M-1 cells with Akt1 knockdown. n, the number of separate M-1 cell lysates.
*P < 0.05 when compared to control non-targeting siRNA-transfected M-1 cells.
|
3. Decreased phospho-AS160 expression in M-1 cells with Akt1 knockdown
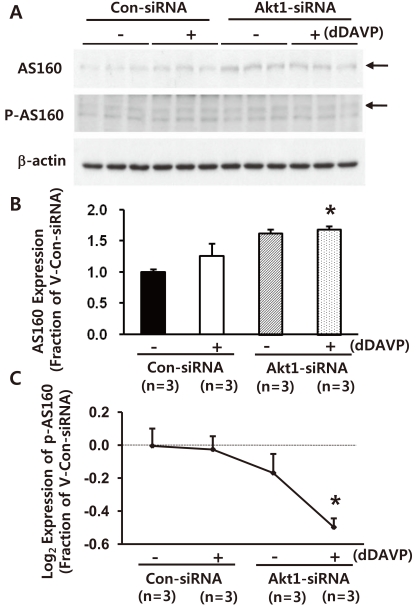 | Fig. 3Semiquantitative immunoblotting of Akt1, AS160, and p-AS160 in mouse cortical collecting duct cells (M-1 cells) with Akt1 knockdown. A-C) Akt1 knockdown was associated with unchanged or marginally increased total AS160 expression. In contrast, phospho-AS160 level normalized to the total AS160 protein values were markedly decreased (~160 kDa band on PAS immunoblots indicated by an arrow in panel A). siRNA, small interfering RNA; n, the number of separate M-1 cell lysates.
*P < 0.05 when compared with the vehicle-treated control group (non-targeting siRNA-transfected M-1 cells).
†P < 0.05 when compared with dDAVP-treated control group (non-targeting siRNA-transfected M-1 cells).
|
4. Increased AQP2 immunolabeling density of the PM in M-1 cells with AS160 knockdown despite the absence of dDAVP stimulation
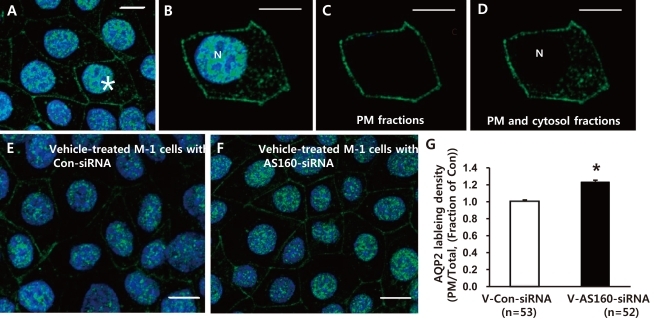 | Fig. 4Semiquantitative immunocytochemistry of aquaporin-2 (AQP2). AQP2-labeled mouse cortical collecting duct cells (M-1 cells) were randomly selected (asterisk in A) and total pixel intensity of the plasma membrane (PM) (C) and whole cell (both PM and cytosol except nucleus, D) were acquired respectively from the selected cells (B). E and F) AQP2 labeling in M-1 cells transfected with Con-small interfering RNA (Con-siRNA) or AS160-siRNA. G) The ratio of total pixel intensity in the PM to that of the whole cell (ratio of PM/Total) was calculated from randomly selected M-1 cells and averaged in each group. N, nucleus; n = number of cells randomly chosen and examined in each group.
*P < 0.05 when compared to Con-siRNA. Bar, 10 µm.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download