Introdution
K+ homeostasis
1. Regulation of K+ between ICF and ECF
1) Driving force
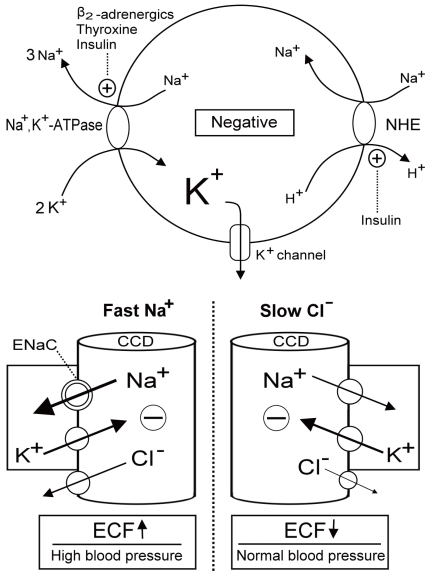 | Fig. 1Regulation of K+ redistribution in cells and K+ secretion in the cortical collecting duct (CCD). The circle depicts the cell membrane (upper panel). Na+,K+-ATPase, Na+/H+ exchanger (NHE), and K+ channels are three major elements controlling K+ shift. Na+,K+-ATPase is activated by β2-adrenergics, insulin and thyroid hormone. NHE, which causes the electroneutral entry of Na+ into cells and thus the net exit of positive voltage via the Na+,K+-ATPase, is also activated by insulin. K+ channels which permit K+ exit is responsible for generating the majority of the resting membrane potential and blocked by barium. The barrel shaped structures represent the terminal CCD (lower panel). The reabsorption of Na+ faster than Cl- (right) or Cl- slower than Na+ (left) in the CCD creates the lumen negative voltage that drives the net secretion of K+.
Fast Na+ disorders cause extracellular fluid (ECF) volume expansion and high blood pressure, whereas, slow Cl- disorders lead to diminished ECF volume and low to normal blood pressure. ENaC, epithelial Na+
channels. |
2) K+ channels
2. Renal handling of K+
1) The flow rate in the CCD
2) [K+]CCD
Aproach to the genetic causes of hypokalemia
1. Disorders with a low urine K+ excretion rate
1) Genetic hypokalemia due to increased K+ shift
(1) Familial hypokalemia periodic paralysis (FPP)
(2) Andersen-Tawil syndrome
2) Genetic hypokalemia where the defect is in the intestinal tract
3) Genetic hypokalemia where the defect is in exocrine glands
2. Disorders with a high urine K+ excretion rate
1) Increased urine flow rate to CCD
2) Increased [K+] in the CCD
(1) Fast Na+ disorders
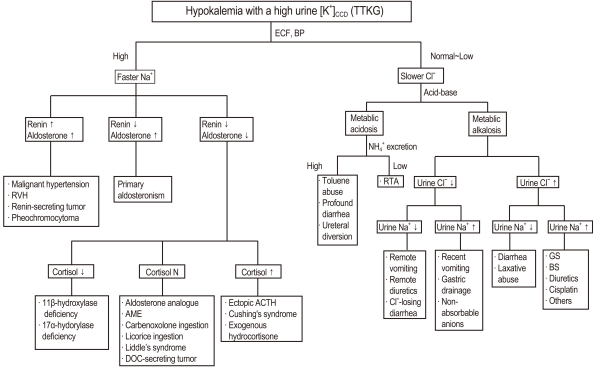 | Fig. 2Algorithm for the approach to hypokalemia with a high urine [K+]CCD (TTKG). CCD, cortical collecting duct; TTKG, transtubular K+ gradient; ECF, extracellular fluid; BP, blood pressure; RVH, right ventricular hypertrophy; AME, Apparent mineralocorticoid excess; DOC, 11-deoxycorticosterone; ACTH, adrenocorticotrophic hormone; RTA, renal tubular acidosis; GS, Gitelman's syndrome; BS, Bartter's syndrome |
a. Genetic hypokalemia associated with mineralocorticoid excess state
i. Glucorticoid-remediable aldosteronism
ii. CAH due to 11β-OHD and 17α-OHD
iii. Liddle's syndrome
iv. Apparent mineralocorticoid excess (AME)
(2) Slow Cl- disorders
a. Hypochloremic metabolic alkalosis
i. Bartter's syndrome (BS) and Gitelman's syndrome (GS)
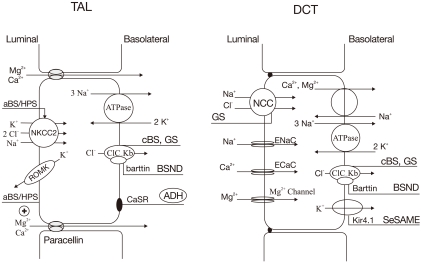 | Fig. 3Transport proteins in the thick ascending limb (TAL) of loop of Henle (LOH) (left panel) and distal convoluted tubule
(DCT) (right panel) affected by gene mutations. Mutations that inactivate Na+/K+/2Cl- cotransporter (NKCC2) or renal outer medullary K+ channel (ROMK) lead to antenatal Bartter syndrome/hyperprostaglandin E syndrome (aBS/HPS) (BS type I and II) and mutations inactivating ClCKb cause classic Bartter syndrome (cBS) (BS type III). Mutations in barttin (chloride channel
βsubunit) cause antenatal BS with sensorineural deafness (BSND) (BS type IV). Mutations that activate the calcium-sensing receptors (CaSR) occur in patients with autosomal dominant hypoparathyroidism (ADH) (BS type V). Mutations inactivating
Na+/Cl- cotransporter (NCC) or basolateral Cl- channel (ClC-Kb) can cause Gitelman's syndrome (GS). Mutations in Kir4.1 channel cause seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME) syndrome. ADH, anti-diuretic hormone |
Table 1
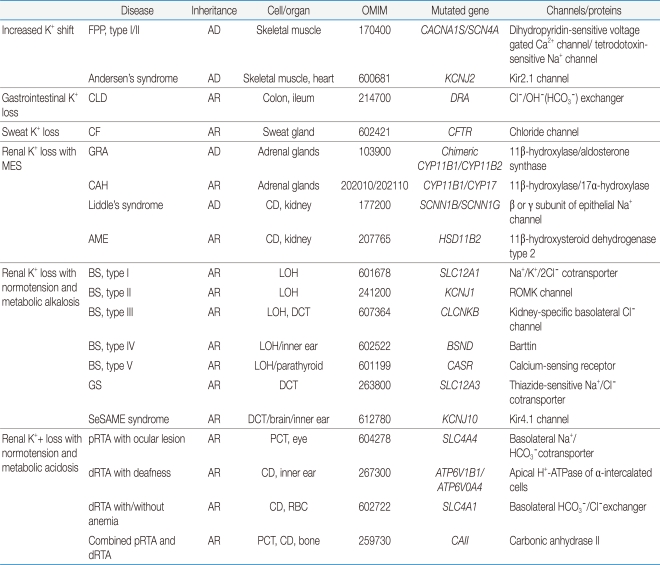
PCT, proximal convoluted tubule; LOH, loop of Henle; DCT, distal convoluted tubule; CD, collecting duct; AD, autosomal dominant; AR, autosomal r ecessive; RBC, red blood cell; GRA, glucocorticoid-remediable aldosteronism; FPP, familial periodic paralysis; CLD, congenital chloride-losing diarrhea; CF, cystic fibrosis; CAH, congenital adrenal hyperplasia; AME, apparent mineralocorticoid excess; BS, Bartter's syndrome; GS, Gitelman's syndrome; pRTA, proximal renal tubular acidosis; dRTA, distal renal tubular acidosis; MES, mineralocorticoid excess state; SeSAME, seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance.
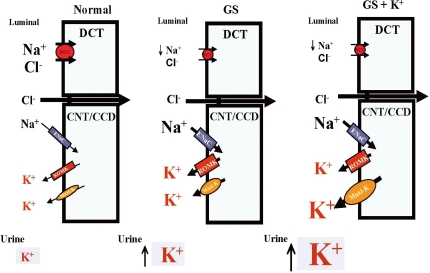 | Fig. 4Mechanisms for persistent hypokalemia in Gitelman's syndrome (GS). Enhanced epithelial Na+ channels (ENaC), renal outer medullary K+ channel (ROMK)1 and Maxi-K expression leads to hypokalemia (middle). High K+ supplement stimulates more accentuated maxi-K expression, accounting for persistent hypokalemia (left). DCT, distal convoluted tubule; CNT, cortical connecting tubules; CCD, cortical colleting duct. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download