Abstract
The impact of glucose-free icodextrin (ID) for overnight dwell as compared to conventional glucose-containing dialysate (GD) on potassium (K+) metabolism in continuous ambulatory peritoneal dialysis (CAPD) patients has not yet been investigated. Serum K+ in a total of 255 stable patients (116 on GD and 139 on ID) on CAPD for more than 6 months and in 139 patients on ID before and after ID use (Pre-ID and Post-ID) were observed along with nutritional markers in a 2-year study period (Jan. 2006 to Dec. 2007). The prevalence of hypokalemia was similar between patients on GD and ID (16.7% vs 17.3%), but was lower on Post-ID than Pre-ID (17.3% vs 20.5%) without statistic significance. The mean serum K+ level was higher on ID than on GD (P<0.05) as well as Post-ID than Pre-ID (P<0.001). In the multivariate analysis, serum K+ levels were positively correlated with serum albumin, and creatinine in all patients (P<0.05), and ID-use in younger patients (age≤56, P<0.001). Serum albumin, creatinine, total CO2, and body mass index were significantly higher on Post-ID than Pre-ID. Icodextrin dialysate for chronic overnight dwell could increase serum K+ levels and lower the prevalence of hypokalemia compared to conventional glucose-containing dialysate. The improved chronic K+ balance in CAPD patients on icodextrin could be related to enhanced nutritional status rather than its impact on acute intracellular K+ redistribution.
The high prevalence of hypokalemia ranging from 10% to 36% has been noticeably observed in continuous ambulatory peritoneal dialysis (CAPD) patients1-3), while hyperkalemia is more prevalent in hemodialysis (HD) patients4). Furthermore, hypokalemic CAPD patients display excess mortality, even after adjusting for multiple confounding factors5).
Serum potassium (K+) concentration is determined by factors influencing both external and internal balance. Hypokalemia in stable CAPD patients may result from inadequate dietary K+ intake and removal of K+ via dialysates, intracellular K+ shift influenced by factors affecting internal balance (such as insulin, catecholamines, aldosterone, and osmolality), or both. Hypokalemia in CAPD patients is a valid surrogate marker for malnutrition, and serum levels of K+ and albumin are well correlated, suggesting that poor dietary K+ intake might derange the external balance5, 6). Although unproven, it has also been proposed that insulin secretion stimulated by continuous intraperitoneal glucose absorption is a contributing factor in deranged internal K+ redistribution7).
Following the introduction of 7.5% icodextrin (ID), a new class of osmotic agents that is an alternative non-glucose-containing dialysate, the clinical benefits of ID have been noted to enhance fluid removal and to improve systemic metabolic derangements such as hyperglycemia, hyperinsulinemia, and muscle wasting8). Although many randomized and observational studies have demonstrated the numerous clinical benefits of ID compared to conventional glucose-containing dialysate (GD)9), the impact of ID on K+ metabolism has not been evaluated. We evaluated differences in serum K+ profiles and influencing factors related to external or internal K+ balance between conventional GD and ID in stable CAPD patients.
Two hundred ninety-two patients (276 at Severance Hospital, Yonsei University, Seoul and 16 at Hanyang University Guri Hospital, Guri, Korea) on CAPD for more than 6 months were initially included for the retrospective study from Jan. 2006 to Dec. 2007. Daily dietary intake of K+ was not restricted in these patients. Thirty-seven patients (20 on GD and 17 on ID) were excluded from the final analysis due to peritonitis (n=11), acute cardiovascular events or severe gastrointestinal symptoms (diarrhea or vomiting) requiring admission (n=11), death (n=6), and conversion to either transplantation (n=5) or hemodialysis (n=4). When divided by the type of dialysate used in the overnight long dwell (8-10 hrs) for the minimum period of 3 consecutive months, 116 patients were on conventional GD (1.5% or 2.5% or 4.5% Dianeal®; Baxter Corporation, Chicago, IL, USA), and the remaining 139 patients were on ID (7.5% glucose-polymer dialysate; Extraneal®, Baxter Corporation, Chicago, IL, USA). Also, to evaluate the differences for ID use, the ID group was divided to pre-ID (before ID use) and post-ID (after ID use) groups.
Hypokalemia was defined as serum K+ level was lower than 3.5 mEq/L. Prevalence of hypokalemia was defined as frequency of one or more events of hypokalemia during 3 consecutive months, not by mean serum K+ level. The blood collections for the biochemical data including serum K+ measurement in the 255 CAPD patients with 4 exchanges of dialysates per day were mostly performed toward the end of each cycle, when they visited the outpatient CAPD clinic for a monthly routine check-up. Mean values of serum level of K+, albumin, creatinine, and total CO2 for the last 3 consecutive months in a 2-year observation period were compared between the two treatment groups (GD vs ID). The use of medication affecting K+ balance such as angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), beta-blockers, K+-sparing diuretics, digoxin, and K+-supplements for more than 1 month was also analyzed.
Serum levels of K+, albumin, creatinine, total CO2, calcium, phosphorus, blood urea nitrogen (BUN), weekly Kt/V, and conventional objective nutritional markers including body weight, body mass index (BMI), total cholesterol, and normalized protein equivalent of total nitrogen appearance (nPNA) were analyzed before ID (Pre-ID) and after ID (Post-ID) treatment in the 139 patients on ID. These parameters were expressed as the mean values of 3 consecutive months on GD just before changing to ID (Pre-ID), and at the end of observation after changing to ID (Post-ID) during the 2-year period. Serum albumin was measured by the bromocresol purple method, and serum creatinine by the Jaffe method. Weekly Kt/V was determined using standard methods10). The nPNA was calculated using the modified Bergstrom formula and normalized by ideal body weight11).
SPSS 12.0.1 for Windows software was used for all statistical analyses (SPSS Inc, Chicago, IL, USA). Descriptive data are expressed as mean±SD (standard deviation). Comparisons of parameters either between GD and ID or between Pre-ID and Post-ID periods were performed by independent or paired t-tests, respectively. Frequency of hypokalemia, among 3 groups, was analyzed by Chi-square test. Correlations between serum K+ and other parameters were evaluated using the Pearson correlation-coefficient. Simple linear and stepwise multiple linear regression analyses were performed for the factors significantly associated with serum K+. A P<0.05 was defined as statistically significant.
Most of the demographic features and baseline characteristics were similar between the GD and ID groups, including sex (M/F, 58/58 vs 66/73), diabetes (21% vs 23%), serum albumin (3.6±0.4 g/dL vs 3.6±0.4 g/dL), serum creatinine (11.0±3.8 mg/dL vs 11.7±4.1 mg/dL), and drugs affecting K+ balance, except for duration of peritoneal dialysis (PD) (85±52 months vs 23±19 months, P<0.05), serum total CO2 (27.7±2.4 mEq/L vs 28.2±2.5 mEq/L, P<0.05), intake of ACEi or ARB. Intake of ACEi or ARB (67% vs 80%, P<0.05) and age (57±13 years vs 54±12 years, P<0.05) were different between the two groups (Table 1).
As shown in Fig. 1, the prevalence of hypokalemia was similar between GD and ID, 16.7% and 17.3%, respectively (P=0.894). However, when the frequency distribution was analyzed in the 139 patients of the ID group before (Pre-ID) and after ID (Post-ID), the prevalence of hypokalemia was higher in Pre-ID (20.5%) than Post-ID (17.3%) without statistical significance (P=0.523). In addition, the mean serum K+ level (range, 1.5-7.3 mEq/L) was higher on ID (4.6±0.7 mEq/L) than on GD (4.4±0.6 mEq/L; P<0.05), and higher in Post-ID (4.6±0.7 mEq/L) than in Pre-ID (4.3±0.7 mEq/L; P<0.001).
In the 255 CAPD patients, serum K+ levels were correlated, using simple linear regression analysis, positively with serum albumin and creatinine, and inversely with age and diabetes. Conversely, serum total CO2, ID-use, and ACEi or ARB use were not correlated with serum K+ levels. In addition, multiple stepwise linear regression analysis revealed that serum K+ levels were correlated with serum albumin and creatinine (Table 2).
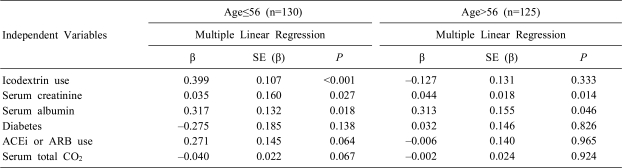
However, when 255 patients were divided into young group (n=130) and old group (n=125) by median age (56 years old) and when multiple stepwise linear regression analysis was performed in each group, serum K+ levels were very significantly correlated with ID-use (also, serum creatinine and albumin) in the young group (Table 3). The results of the old group were similar to the total group (Table 2).
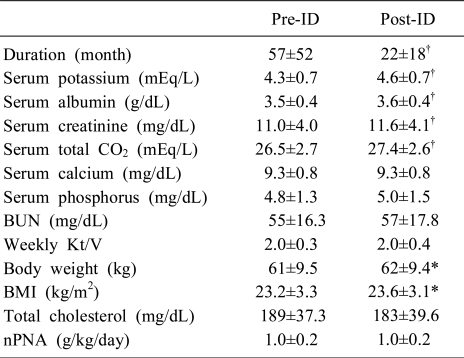
In 56 patients from the ID group (n=139), in whose data were possible to perform the paired t-test, serum albumin (3.6±0.4 g/dL vs 3.5±0.4 g/dL), serum creatinine (11.6±4.1 mg/dL vs 11.0±4.0 mg/dL), serum total CO2 (27.4±2.6 mEq/L vs 26.5±2.7 mEq/L), body weight (62±9.4 kg vs 61±9.5 kg), and BMI (23.6±3.1 vs 23.2±3.3) were significantly higher in the Post-ID than Pre-ID group. Duration of PD was significantly shorter in Post-ID than Pre-ID (22±18 months vs 57±52 months, P<0.001). Otherwise, serum calcium (9.3±0.8 mg/dL vs 9.3±0.8 mg/dL), serum phosphorus (4.8±1.3 mg/dL vs 5.0±1.5 mg/dL), BUN (55±16.3 mg/dL vs 57±17.8 mg/dL), weekly Kt/V (2.0±0.3 vs 2.0±0.4), total cholesterol (189±37.3 mg/dL vs 183±39.6 mg/dL), and nPNA (1.0±0.2 g/kg/day vs 1.0±0.2 g/kg/day) were similar in both Pre-ID and Post-ID groups (Table 4).
In the present study, the mean serum K+ levels were significantly higher for 3 consecutive months on ID than on either GD (P<0.05) or Pre-ID (P<0.001) (Table 1, 4). In addition, the prevalence of hypokalemia in the ID subgroup decreased from 20.5% on GD to 17.3% after changing to ID, although the distribution of serum K+ in CAPD patients on conventional GD and ID was comparable between the two groups.
To the best of our knowledge, this study is the first to analyze the difference in the frequency of serum K+ distribution in chronic stable CAPD patients on conventional GD versus ID for a long dwell at night time, and on the potential causes of any identified differences. Consistent with previous reports, the prevalence of hypokalemia was above 10% in both GD and ID patients1-3). However, switching from GD to ID improved serum K+ profiles and increased the frequency of normokalemia, which was accompanied by significant improvements in nutritional status, such as BMI, and biochemical markers of nutrition such as serum albumin and creatinine (Table 4). In addition, serum K+ levels were positively associated with serum albumin and creatinine in the total group and were significantly associated with ID-use in the young group (Table 2, 3). Taken together, these results demonstrate that the improved serum K+ profiles observed in young CAPD patients on ID were accompanied by the enhancement of some important nutritional markers.
Homeostasis of K+ can be maintained by the integrated activities of both transcellular K+ redistribution or shifting (internal balance) and dietary intake or external losses through the gastrointestinal tract, skin, or kidneys (external balance)7). In the state of negligible renal K+ excretion and a fixed amount of fecal loss in end stage renal disease patients, the amount of K+ removed by CAPD is determined by the instillation and ultrafiltration volumes as well as the type of dialysate, the number of daily exchanges, and the serum K+ level of the patient. Under otherwise stable medical conditions in CAPD patients with four daily exchanges of conventional GD, K+ removal via dialysis equals about 25-30 mEq of K+ per day, which is significantly lower than the 60-90 mEq of usual daily K+ intake on the western diet1, 12). Most CAPD patients have normokalemia, but some are still prone to develop hypokalemia1, 4).
In stable CAPD patients without excessive stool losses, hypokalemia presumably develops due to either decreased dietary K+ intake or increased dialysis adequacy. Raising peritoneal dialysis adequacy targets to Kt/V per week > 2.0 leads to a rise in the frequency of hypokalemia and a greater number of CAPD patients requiring K+ supplementation12). In this study, however, weekly Kt/V was equal before (Pre-ID) and after ID (Post-ID), in spite of a significantly higher prevalence of hypokalemia in the period on GD before changing to ID (Pre-ID). This finding suggests that the adequacy of dialysis may be irrelevant in influencing the prevalence of hypokalemia.
A different balance study in a small group of four CAPD patients with hypokalemia who required persistent K+ supplements revealed positive external K+ balances7). The amount of K+ in dietary K+ intake and oral supplements minus the amount removed by urine and dialysate was positive. The authors suggested, as a possible hypothesis for hypokalemia in CAPD patients, that the internal K+ balance was broken (i.e., K+ redistribution into the intracellular compartment) by the excessive release of insulin with continuous glucose absorption through the peritoneal membrane7). In reality, the K+ content in muscles of CAPD patients is elevated, indicating that K+ stores are well maintained in the intracellular compartment by the intracellular shift of K+ promoted by a higher insulin level in response to peritoneal glucose absorption, despite continuous removal of K+ on CAPD7, 13). However, in our previous balanced study of acute intraperitoneal K+ load in nine stable CAPD patients on conventional GD or ID, we observed that the plasma [K+] increment was significantly high in ID compared to GD after K+ load and the increment of insulin was significantly lower in ID than in GD14). Thus glucose containing dialysate could induce insulin secretion that caused K+ redistribution toward intracellular space14). Although we only evaluated nine CAPD patients, the result of seven diabetic PD patients in our study has shown that the increment of insulin was significantly high in GD compared to ID, after K+ load14). Such results represented that insulin, also in diabetic end-stage renal disease (ESRD) patients, could play a role in controlling internal K+ balance14).
Hypokalemia, a somewhat frequent disorder in patients on chronic CAPD in this study, seemed to be related to low dietary K+ intake for an extended period, making malnutrition an important contributing or associated factor. Recent reports have shown that hypokalemia on CAPD is associated with nutritional indicators such as serum albumin level, PNA, and BMI6). Furthermore, serum K+ levels in Chinese CAPD patients are associated with nutritional status and severity of comorbid conditions5). The hypokalemia seems to most likely be a surrogate marker of malnutrition combined with poor dietary intake of K+. The high prevalence of hypokalemia in CAPD patients, which is consistent with the high prevalence of malnutrition in CAPD patients on conventional GD, has been noted as 18-56% in numerous previous reports15, 16).
When hypokalemic pediatric CAPD patients increase their dietary K+ intake by a small amount (11-64%) without any other K+ supplementation, serum K+ levels return to the normal range, suggesting that hypokalemia may be correctable through dietary counseling to increase K+ intake17). Even so, the chronic management of hypokalemia in stable CAPD patients may require improvement of nutritional status on a long-term basis rather than simple correction by K+ supplementation on a short-term basis through either oral routes, such as a K+-rich diet and oral K+ preparation, or intraperitoneal routes3, 17). Increased dietary protein intake with multidisciplinary intervention represents an appropriate approach for chronic management of hypokalemia in CAPD patients with malnutrition, since dietary protein contains about 1 mEq of K+ per gram18). In our analysis, however, nPNA (a known indicator of dietary protein intake) was similar between the Post-ID (on ID) and the Pre-ID (on GD) periods, despite higher Post-ID serum K+ levels. The reliability of nPNA as an indicator has been questioned, particularly in hypercatabolic cases with malnutrition19).
ID, a non-glucose-containing polymer-based PD solution, was originally produced to treat ultrafiltration failure, particularly that caused by rapid glucose absorption due to the high transport type of peritoneal membranes in CAPD patients20). ID has been widely used for single nighttime long dwells, resulting in better fluid status and blood pressure control in CAPD patients with high peritoneal membrane permeability (because of the increase in ultrafiltration volume), enhanced quality of life and patient survival21), and biochemical benefits including improved lipid profiles and insulin sensitivity22, 23).
Short-term treatment with ID for 1 week is associated with a greater loss of amino acids from dialysate, thus, long term treatment with ID could have a detrimental effect on nutritional status. The greater loss of amino acids might merely reflect the extra loss of amino acids during a daytime dwell. In reality, plasma levels of amino acids do not change, indicating a lower probability of nutritional deficits on ID24). Furthermore, in view of the proportionately greater muscle wasting and increased fat accumulation in CAPD patients maintained on PD regimens with a higher exposure to glucose, those with lower glucose exposure and sustained use of ID for daily long dwells might experience beneficial effects on their nutritional status.
We observed that ID improved some nutritional markers compared to conventional GD in CAPD patients, which were associated with better serum K+ profiles. There are a number of possible explanations for these benefits of ID. First, the increase of serum K+ levels seen with ID could be related to improved volume status, as supported by the observation that serum K+ levels are positively correlated with ultrafiltration volume in peritoneal equilibration tests6). Second, hypokalemia in CAPD patients has been proposed as a surrogate marker for malnutrition or severe underlying comorbid conditions, which are both related to poor dietary K+ intake5). Third, the hyperglycemia and delayed gastric emptying with reduced gastrointestinal motility seen in conventional GD is improved by the avoidance of glucose in ID, which could lead to improved nutritional status and a higher dietary K+ intake25, 26). Finally, ID does not affect appetite, while anorexia is frequently seen in CAPD patients on conventional GD27).
This study was limited by its retrospective and cross-sectional design. The impact of the effluent dialysate of ID on the nighttime long dwell on the amount of K+ removed was not considered on a long-term basis and also, the data of residual renal function and peritoneal function were not considered for analysis. Moreover, only limited data from 3 consecutive months in CAPD patients were included in this study and multivariable analyses of nutritional markers for ID-use were not performed due to small sample size. In addition, a detailed dietary assessment was not available in this study. Finally, although serum albumin was measured in this study, other common nutrition parameters, such as assessment of intake and subjective global assessment (SGA), and anthropometric assessments, such as skin fold thickness and mid-arm muscle circumference, were not evaluated28).
In conclusion, the increase in serum K+ level after switching from GD to ID, and the higher levels of serum K+ while on ID versus GD, indicate that ID represents a viable option for managing chronically hypokalemic patients on CAPD. Serum K+ was positively correlated with nutritional factors such as serum albumin level in all patients and in the ID group, implying that poor nutrition and K+ intake might contribute significantly to hypokalemia in CAPD patients.
References
1. Oreopoulos DG, Khanna R, Williams P, Vas SI. Continuous ambulatory peritoneal dialysis - 1981. Nephron. 1982; 30:293–303. PMID: 7110460.

2. Khan AN, Bernardini J, Johnston JR, Piraino B. Hypokalemia in peritoneal dialysis patients. Perit Dial Int. 1996; 16:652. PMID: 8981546.

3. Spital A, Sterns RH. Potassium supplementation via the dialysate in continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1985; 6:173–176. PMID: 3898826.

4. Tzamaloukas AH, Avasthi PS. Temporal profile of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am J Nephrol. 1987; 7:101–109. PMID: 3605230.

5. Szeto CC, Chow KM, Kwan BC, et al. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis. 2005; 46:128–135. PMID: 15983966.

6. Kim HW, Chang JH, Park SY, et al. Factors associated with hypokalemia in continuous ambulatory peritoneal dialysis patients. Electrolyte Blood Press. 2007; 5:102–110.

7. Tziviskou E, Musso C, Bellizzi V, et al. Prevalence and pathogenesis of hypokalemia in patients on chronic peritoneal dialysis: one center's experience and review of the literature. Int Urol Nephrol. 2003; 35:429–434. PMID: 15160552.

8. McIntyre CW. Update on peritoneal dialysis solutions. Kidney Int. 2007; 71:486–490. PMID: 17299524.

9. Davies SJ. Exploring new evidence of the clinical benefits of icodextrin solutions. Nephrol Dial Transplant. 2006; 21(Suppl 2):ii47–ii50. PMID: 16825261.

10. Nolph KD, Moore HL, Twardowski ZJ, et al. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J. 1992; 38:M139–M142. PMID: 1457833.

11. Bergstrom J, Heimburger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int. 1998; 18:467–473. PMID: 9848623.

12. Newman LN, Weiss MF, Berger J, Priester A, Negrea LA, Cacho CP. The law of unintended consequences in action: increase in incidence of hypokalemia with improved adequacy of dialysis. Adv Perit Dial. 2000; 16:134–137. PMID: 11045278.
13. Bergstrom J, Alvestrand A, Furst P, Hultman E, Widstam-Attorps U. Muscle intracellular electrolytes in patients with chronic uremia. Kidney Int. 1983; 24(Suppl 16):S153–S160.
14. Yi JH, Park JI, Choi HY, Lee HY, Han SW, Kim HJ. Comparison of icodextrin and 2.5% glucose in potassium metabolism by acute K+ load via dialysate in continuous ambulatory peritoneal dialysis patients. Electrolyte Blood Press. 2009; 7:25–30.
15. Kang DH, Kang EW, Choi SR, Yoon SY, Han DS. Nutritional problems of Asian peritoneal dialysis patients. Perit Dial Int. 2003; 23(Suppl 2):S58–S64. PMID: 17986560.

16. Young GA, Kopple JD, Lindholm B, et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: an international study. Am J Kidney Dis. 1991; 17:462–471. PMID: 1901197.

17. Factor KF. Potassium management in pediatric peritoneal dialysis patients: can a diet with increased potassium maintain a normal serum potassium without a potassium supplement? Adv Perit Dial. 2007; 23:167–169. PMID: 17886626.
18. Messa P, Mioni G, Maio GD, et al. Derangement of acid-base balance in uremia and under hemodialysis. J Nephrol. 2001; 14(Suppl 4):S12–S21. PMID: 11798141.
19. Harty JC, Boulton H, Curwell J, et al. The normalized protein catabolic rate is a flawed marker of nutrition in CAPD patients. Kidney Int. 1994; 45:103–109. PMID: 8126998.

20. Peers E, Gokal R. Icodextrin: overview of clinical experience. Perit Dial Int. 1997; 17:22–26. PMID: 9068018.

21. Kuriyama R, Tranaeus A, Ikegami T. Icodextrin reduces mortality and the drop-out rate in Japanese peritoneal dialysis patients. Adv Perit Dial. 2006; 22:108–110. PMID: 16983951.
22. Bredie SJ, Bosch FH, Demacker PN, Stalenhoef AF, van Leusen R. Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Perit Dial Int. 2001; 21:275–281. PMID: 11475343.

23. Gursu EM, Ozdemir A, Yalinbas B, et al. The effect of icodextrin and glucose-containing solutions on insulin resistance in CAPD patients. Clin Nephrol. 2006; 66:263–268. PMID: 17063993.
24. van Hoeck KJ, Rusthoven E, Vermeylen L, et al. Nutritional effects of increasing dialysis dose by adding an icodextrin daytime dwell to Nocturnal Intermittent Peritoneal Dialysis (NIPD) in children. Nephrol Dial Transplant. 2003; 18:1383–1387. PMID: 12808177.

25. Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int. 2003; 64:1480–1486. PMID: 12969169.

26. Van V, Schoonjans RS, Struijk DG, et al. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int. 2002; 22:32–38. PMID: 11929141.

27. Zheng ZH, Sederholm F, Anderstam B, et al. Acute effects of peritoneal dialysis solutions on appetite in non-uremic rats. Kidney Int. 2001; 60:2392–2398. PMID: 11737615.

28. Steinman TI. Serum albumin: its significance in patients with ESRD. Semin Dial. 2000; 13:404–408. PMID: 11130266.
Fig. 1
The prevalence of hypokalemia between patients treated with glucose-containing dialysate (GD, n=116) and icodextrin (ID, n=139), and before (Pre-ID) and after (Post-ID) changing to icodextrin in 139 patients on icodextrin (ID). GD, glucose-containing dialysate; ID, non-glucose-containing dialysate, icodextrin; Pre-ID, before icodextrin, i.e. on GD; Post-ID, after icodextrin.
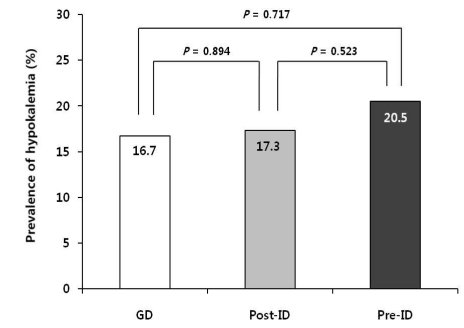
Table 1
Demographic and Baseline Serum Biochemical Values of 255 Patients on CAPD Divided according to Their Dialysate Type on Overnight Long Dwell
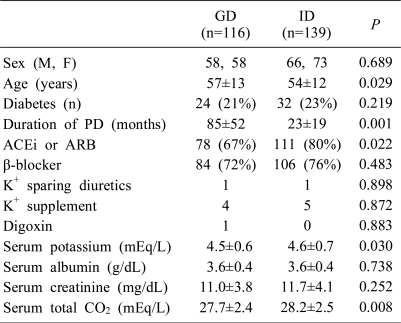
Values are expressed as mean±SD (standard deviation) or number of patients (%). CAPD, continuous ambulatory peritoneal dialysis; GD, glucose-containing dialysate; ID, non-glucose-containing dialysate, icodextrin; PD, peritoneal dialysis; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor antagonist; K+, potassium.
Table 2
Correlation between Serum Potassium Levels and Other Parameters in 255 Patients on CAPD Analyzed by Simple and Multiple Stepwise Linear Regression
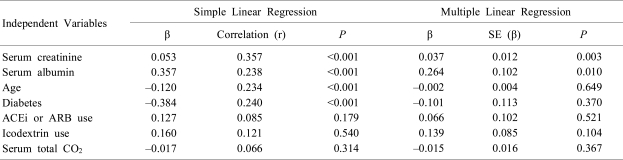
Table 3
Correlation between Serum Potassium Levels and Other Parameters in Young Group (≤56 years old) and Old Group (>56 years old) Analyzed by Multiple Stepwise Linear Regression




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download