Introduction
Continuous ambulatory peritoneal dialysis (CAPD) patients using conventional glucose-containing dialysates (GD) reveal a high prevalence of hypokalemia
1-
4). Spital et al. documented that 36% of all CAPD patients had hypokalemia, that about 20% of these patients received potassium (K
+) supplement, and for the correction of acute hypokalemia, a method using dialysate directly mixed with K
+ was very safe and effective
3). Furthermore, in a recently reported Chinese study, hypokalemia, represented in 20.3% of CAPD patients, could be an independent risk factor for their survival
5).
7.5% icodextrin (ID), a new class of osmotic agents that is an alternative non-glucose-containing dialysate, has now been proven to be clinically useful in the fluid management of peritoneal dialysis (PD) patients and has improved biocompatibility when compared to the traditional solutions
6-
9). We evaluated differences in serum K
+ profiles and influencing factors related internal K
+ balance between GD and ID in stable CAPD patients, and additionally the safety and effectiveness of acute intraperitoneal K
+ load.
Go to :

Subjects and Method
1. Subjects
We enrolled nine stable CAPD patients at Hanyang University Guri Hospital. They only used 1.5% or 2.5% glucose-containing peritoneal dialysate (GD, Dianeal®, Baxter Corporation, Chicago, Illinois, USA) 2 L regularly exchanged 4 times per day and were prescribed a strict low K+ diet (0.8 mEq/kg/day) by a renal dietician for at least 2 months. We excluded the patient who had received oral hypoglycemic agents. This interventional study was approved by the internal review board of Hanyang University Guri Hospital, and individual written consent was obtained before the study.
2. Methods
All enrolled patients received a 6-hr dwell of 2 L of 2.5% GD mixed with 40 mEq (20 mEq/L) of potassium chloride (KCl) on fasting in the morning. The same method was repeated with ID dialysate after a one-week interval. They stopped insulin therapy and β-blockers on the day of the intraperitoneal K+ load test.
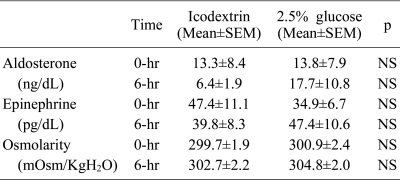
Blood and peritoneal dialysate samplings were performed just before dialysate inflow and right after dialysate outflow in order to evaluate acute changes in serum K
+, serum osmolality and neurohormones (including epinephrine, insulin, and aldosterone levels) after the peritoneal K
+ loads. The total amounts of K
+ absorbed from K
+-mixed dialysate during dialysis and of K
+ translocated into cells were calculated by the equation (1) of Spital et al.
3).
(1) Calculated amount (%) of K
+ shifted to ICF = [(K
+ absorbed - 0.2 × BW × Δ serum K
+) ÷ K
+ absorbed]×100%
3)
ICF, intracellular fluid; BW, body weight
Plasma insulin and serum aldosterone levels were measured by radioimmunoassay (insulin kit by Eiken Co., Tokyo, Japan, and aldosterone kit by Abbot Laboratories, Wiesbaden, Germany), plasma epinephrine by high-performance liquid chromatography and electrochemical detection with a Waters 460 electrochemical detector (Waters Co., Milford, MA, USA), and serum osmolality with a Vapor Pressure Osmometer (Wescor Inc., Logan, UT, USA).
3. Statistical analysis
SPSS 12.0.1 for Windows software was used for all statistical analyses (SPSS Inc, Chicago, IL, USA). Descriptive data are expressed as mean±SD. The comparison between ID and GD was assessed by Wilcoxon Signed Ranks Test. A p value<0.05 was defined as statistically significant.
Go to :

Discussion
Generally, K
+ homeostasis is controlled by external and internal K
+ balance. External K
+ balance is maintained between K
+ intake and excretion through the kidney and intestine. Internal K
+ balance is affected by K
+ redistribution between intracellular and extracellular spaces. However, in ESRD, K
+ excretion through kidney reduces, thus, K
+ homeostasis is maintained by low K
+ diet, K
+ removal by dialysis and intestine, and internal balance
10). Especially, K
+ removal in PD occurs by dialysate and ultrafiltrate amounts, exchange times per day, and average plasma [K
+] level
10,
11). Theoretically it would not be enough to maintain a safe plasma K
+ level because daily K
+ intake is greater than daily K
+ removal through PD and excretion via the intestine
1,
10). However, actually, in PD patients, normokalemia and mild hypokalemia are more frequent than hyperkalemia
1-
4). Why does that phenomenon occur? The answers are that K
+ moves in intracellular space as glucose containing dialysate stimulates insulin secretion and intracellular and intramuscular Kv levels are higher in PD patients than in HD patients
12). Thus, internal K
+ balance, so called K
+ redistribution, could play a role to maintain K
+ homeostasis in PD.
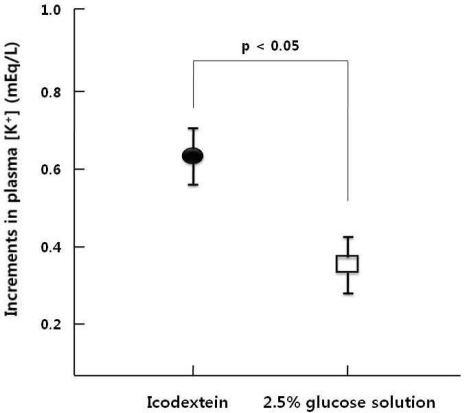
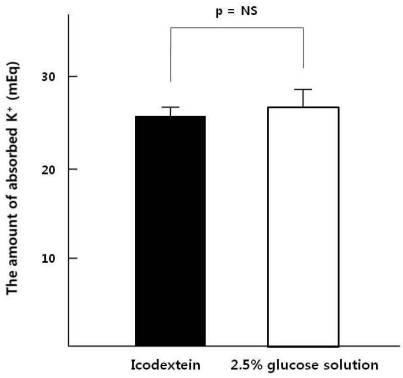
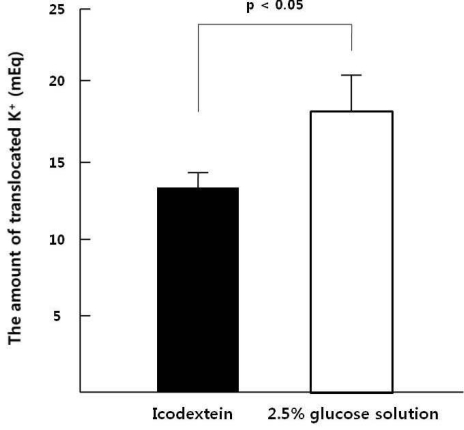
After acute peritoneal K
+ load, K
+ absorption rates through the peritoneum were similar between ID and GD (65±2% vs. 68±2%), However Spital et al. reported that K
+ absorption rates through the peritoneum were 73%
3). The difference of absorption rates can be explained by the residual renal K
+ excretion that might slightly remain in our study population because the average urine volume was 602 mL/day. Plasma [K
+] increment was significantly higher in ID than GD after K
+ load and the increment of insulin was significantly lower in ID than GD. Thus glucose containing dialysate could induce insulin secretion that caused K
+ redistribution toward intracellular space.
Furthermore, a noticeable point is that internal K
+ balance for K
+ homeostasis could be intact in diabetic ESRD patients who have insulin resistance and impairment of insulin secretion
3). A previous study by Tzamalouksa et al. has shown that the frequency and causes for plasma [K
+] disturbances are similar between diabetic and nondiabetic dialysis patients
10). A critical limitation of this study is that the size of our study population was not large enough to compare the difference between diabetic and nondiabetic PD patients, the result of seven diabetic PD patients in our study has shown that the increment of insulin was significantly higher in GD than ID, after K
+ load. Such results represented that insulin, also in diabetic ESRD patients, could play a role to control internal K
+ balance.
As mentioned above, the long term use of high-glucose containing dialysate could provoke peritoneal damage and insulin secretion that causes hyperinsulinemia or atherosclerotic cardiovascular accidents
13). On the other hand, ID, as iso-osmolar and starch-derived glucose polymer, reduces hyperinsulinemia, insulin resistance
13), and glucose degradation product (GDP) which material causes the peritoneal damage by the stimulation of mesothelial cell apoptosis and oncosis in peritoneum
14). One study reported that the long term use of ID does not cause weight gain and edema
15). Thus ID is expected to substitute for GD in hypokalemic PD patients due to lower peritoneal damage and lower K
+ shift to intracellular space than GD.
In conclusion, we suggest that intraperitoneal K+ load and using ID to substitute for GD, as the treatment for acute hypokalemia in CAPD patient, could be safe and effective methods because ID less stimulates insulin secretion than GD. K+ homeostasis, in diabetic CAPD patients, could be well controlled without defects of internal K+ balance, so called K+ redistribution, in this study.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download