Abstract
Pendrin (SLC26A4) is a Na+-independent Cl-/HCO3- exchanger which is expressed in the apical membranes of type B and non-A, non-B intercalated cells within the distal convoluted tubule, the connecting tubule, and the cortical collecting duct. In those segments it mediates HCO3- secretion and chloride (Cl-) absorption. In mice, no renal abnormalities are observed under basal conditions, and individuals with genetic disruption of the pendrin (SLC26A4) gene (Pendred syndrome) have normal acid-base balance. In contrast, there are definite differences under conditions wherein the transporter is stimulated. In animal studies, pendrin (SLC26A4) is upregulated with aldosterone analogues, Cl- restriction, and metabolic alkalosis, and is down-regulated with Cl loading and metabolic acidosis, independently. However, the exact role of pendrin in humans has not been established to date, and further examinations are necessary.
The kidney is one of the major organs that maintain acid-base homeostasis via three major processes. In the proximal tubule and the thick ascending limb, filtered bicarbonate (HCO3-) is reabsorbed, and acid/protons are excreted in the collecting duct. The other process includes the synthesis of ammonia and the use of ammonia, phosphate and citrate as so-called titratable acids to bind protons and thereby maximizing the kidneys ability to excrete proton. These processes of the kidney to excrete acids or bases are associated with the expression of various specialized and spatially arranged transport proteins and enzymes.
Genes responsible for some inherited disorders of renal acid-base transport have been identified1). They can provide molecular mechanisms for understanding the role of renal transporters in acid-base regulation. For example, mutations in gene encoding the Na-/HCO3--cotransporter NBCe1 (SLC4A4) cause defects of HCO3- absorption in the proximal tubule, leading to proximal renal tubular acidosis. Patients suffer from severe metabolic acidosis and blindness since the transporter is not only expressed on the basolateral side of the proximal tubule but also in the eye2). Mutations in the cytosolic carbonic anhydrase (CAII) gene cause a mixed type of proximal and distal renal tubular acidosis because CAII expresses in the proximal and distal tubules. Patients also suffer from cerebral calcification and osteopetrosis. Inherited forms of distal renal tubular acidosis could be caused by mutations of the vacuolar H--ATPase or the Cl-/HCO3- exchanger, anion exchanger 1 (AE1)1). These patients' manifestations include hyperchloremic metabolic acidosis and hypokalemia and are often associated with retarded growth in infants or nephrocalcinosis and nephrolithiasis in adults.
Over the past 20 years, the importance of intercalated cells in the process of renal net acid excretion has been recognized. The final control of urinary acidification occurs in the late distal tubule, the connecting segment and the collecting duct through the action of intercalated cells3). Intercalated cells represent the minority cell type within the connecting tubule (CNT), the distal convoluted tubule (DCT) and the cortical collecting ducts (CCD). Intercalated cells are classified as type A, type B, or non-A, non-B based on the presence or absence of the anion exchanger, AE1, and the distribution of the H+-ATPase within the cell4, 5). Intercalated cells excrete or reabsorb net H+ equivalents depending on whether the H+-ATPase is expressed on the apical or the basolateral membrane. Type A intercalated cells secrete H+ equivalents through the H+-ATPase which localizes to the apical membrane and functions in series with the Cl-/HCO3- exchanger, AE1 expressed on the basolateral membrane. In the type B intercalated cells (Cl-/HCO3- exchanger), pendrin, across the apical membrane, functions in series with the H+-ATPase expressed on the basolateral membrane to mediate secretion of OH- equivalents (Fig. 1).
In human, mouse and rat kidney, pendrin is expressed in the CCD, the CNT, the initial collecting tubule and the DCT, where it localizes to the apical plasma membrane and apical cytoplasmic vesicles of both type B and non-A, non-B IC6, 7). Apical localization of pendrin to a subset of intercalated cells suggests that pendrin participates in the regulation of acid-base balance. However, intercalated cells also participate in the process of chloride (Cl-) absorption and secretion.
Recent research suggest that pendrin is important in protecting the body from the development of metabolic alkalosis as evident from pendrin-deficient mice8). In human cases, Pendred syndrome is caused by the mutations of pendrin. In 1896 Vaughan Pendred first described an Irish family in which 2 of 10 children suffered from congenital deafness and goiter. Everett et al.9) reported the molecular structure of pendrin (SLC26A4). The solute carrier family 26 (SLC26) is a group of multifunctional anion transporters, which play a physiological role in almost all organ systems, functioning in the transport of anions such as sulfate, iodide, formate, oxalate, Cl-, hydroxyl and HCO3-10). SLC26-dependent transport of hydroxyl and HCO3- anions impacts on the regulation of intracellular and plasma pH. Pendrin (SLC26A4) is one of the well-known members of the SLC26 family in the kidney. However, the physiological significance of pendrin in the human kidney is not obvious since affected individuals have no known acidbase or fluid and electrolyte abnormalities, and normal renal function is present at least under basal and unstimulated conditions.
Pendrin activity is controlled on at least four different levels, including mRNA expression and protein abundance, subcellular localization, and numbers of pendrin expressing non type-A intercalated cells (Fig. 2)11). Adler et al.12) indicated that pendrin (SLC26A4) has direct regulatory domains in the promoter region sensitive to intracellular pH and possibly to aldosterone. Several treatments, including acidosis and alkalosis, altered total pendrin abundance in the kidney13, 14). Subcellular localization of pendrin in animal models was observed15). Lastly, the number of pendrin expressing non-type-A intercalated cells was observed in states of chronic metabolic acidosis or altered distal Cl- delivery associated with a changed relative abundance of pendrin positive cells14, 15). Through these mechanisms, altered pendrin activity could be observed in various animals under specific stimuli.
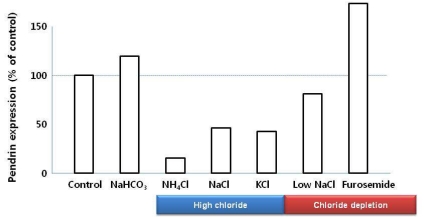
Pendrin mediates Cl-/HCO3- exchange, which releases HCO3- into urine and reabsorbs Cl-. During metabolic acidosis, pendrin expression has been down-regulated13, 14, 16). Pendrin is also important for Cl- reabsorption in the CNT and CCD, which are pendrin-rich segments. Increased Cl- delivery is associated with reduced pendrin expression levels (Fig. 3)16). However, Hafner et al.14) emphasized that metabolic acidosis caused by acetazolamide or (NH4)2SO4 loading prevents the increase or even decreased pendrin expression despite low level of urinary Cl- excretion, which suggests pendrin is regulated by acid-base status independent of Cl- status.
During metabolic alkalosis induced by HCO3--loading, pendrin abundance was increased13, 15). Potassium depletion also caused reduced protein levels of pendrin, which suggests the negative effect of metabolic alkalosis during K- restriction on pendrin expression13, 15). Verlander et al.8) showed that pendrin is upregulated with aldosterone analogues (deoxycorticosterone pivalate, DOCP) and that pendrin is critical in the pathogenesis of mineralocorticoid-induced hypertension and metabolic alkalosis. However, it is not entirely clear if DOCP has a direct effect on pendrin or an indirect effect via hypokalemia or metabolic alkalosis.
Experimental studies using pendrin null mice showed the functional role of pendrin under various conditions8, 17, 18). Royaux et al.17) reported that pendrin knock-out mice receiving DOCP in addition to NaHCO3 loading failed to secrete HCO3-. Wall et al.18) indicated that pendrin (SLC26A4) is upregulated with NaCl restriction and is critical in the maintenance of acid-base balance and in the renal conservation of Cl- and water during NaCl restriction via pendrin (SLC26A4) null mice under NaCl restriction diet. Recently, Pela et al.19) reported that a 5-year-old child with Pendred syndrome whom had developed profound hypokalemia and severe hypochloremic metabolic alkalosis following thiazide therapy. They suggested that the improper action of pendrin may exacerbate the diuretic effects of thiazide, which inhibits Cl- reabsorption mediated by the thiazide-sensitive NaCl cotransporter19).
Pendrin is a Na+-independent, Cl-/HCO3- exchanger that is expressed in the apical membranes of type B and non-A, non-B intercalated cells, which mediates HCO3- secretion and Cl- absorption. Under basal conditions, no renal abnormalities are observed in mice and humans with genetic disruption of Pendred syndrome. In contrast, pendrin (SLC26A4) is upregulated with aldosterone analogues, Cl- restriction, and metabolic alkalosis, and is down-regulated with Cl- loading and metabolic acidosis, independently. However, the exact role of pendrin in humans has not been established to date, and further examinations are necessary.
References
1. Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol. 2002; 64:899–923. PMID: 11826292.

2. Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, et al. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004; 279:52238–52246. PMID: 15471865.

3. Wagner CA, Geibel JP. Acid-base transport in the collecting duct. J Nephrol. 2002; 15(Suppl 5):S112–S127. PMID: 12027210.
4. Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA. 1989; 86:5429–5433. PMID: 2526338.
5. Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol. 1993; 55:267–288. PMID: 8466177.

6. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol. 2002; 283:F744–F754. PMID: 12217866.
7. Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, et al. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003; 284:F229–F241. PMID: 12388426.

8. Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003; 42:356–362. PMID: 12925556.
9. Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997; 17:411–422. PMID: 9398842.

10. Sindic A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007; 16:484–490. PMID: 17693766.
11. Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflugers Arch. 2009; 458:137–156. PMID: 19277700.

12. Adler L, Efrati E, Zelikovic I. Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol. 2008; 294:C1261–C1276. PMID: 18322141.

13. Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol. 2003; 284:F584–F593. PMID: 12556366.
14. Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Clexcretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol. 2008; 295:C1658–C1667. PMID: 18971389.

15. Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, et al. Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 2002; 62:2109–2117. PMID: 12427135.

16. Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, et al. The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol. 2004; 287:F1179–F1188. PMID: 15292050.
17. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001; 98:4221–4226. PMID: 11274445.

18. Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension. 2004; 44:982–987. PMID: 15477386.
19. Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008; 69:450–453. PMID: 18538122.

Fig. 1
Classification of intercalated cell subtypes. Pendrin is expressed on the apical membranes of type B intercalated cells and non-A, non-B intercalated cells.
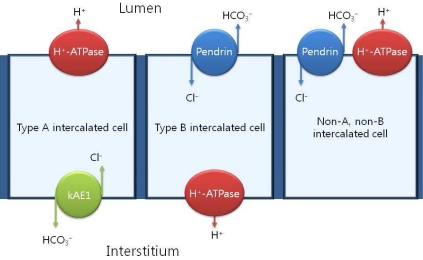
Fig. 2
Pendrin activity in 4 different levels. Pendrin activity is controlled on at least 4 different levels, including mRNA expression and protein abundance, subcellular localization, and numbers of pendrin expressing non type-A intercalated cells.
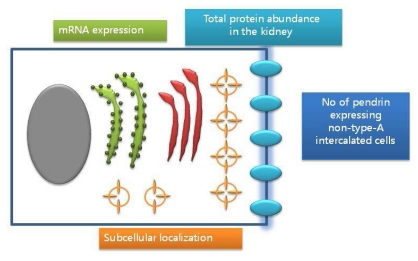
Fig. 3
Effects of NaHCO3 loading, high chloride loading (NH4Cl, NaCl, and KCl), and chloride depletion (low NaCl, and Furosemide loading) on pendrin expression. Pendrin expression is inversely related to diet-induced changes in urinary chloride excretion independent of the administered cation13).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download