Abstract
Ethylene glycol is a widely used and readily available substance. Ethylene glycol ingestion does not cause direct toxicity; however, its metabolites are highly toxic and can be fatal even in trace amounts. Poisoning is best diagnosed through inquiry, but as an impaired state of consciousness is observed in most cases, poisoning must be suspected when a significantly elevated osmolar gap or high anion gap metabolic acidosis is found in blood tests. Hemodialysis and alcohol dehydrogenase inhibitors such as ethanol and fomepizole are a part of the basic treatment, and timely diagnosis and treatment are crucial because any delays can lead to death. However, there are few reported cases in Korea, and no report on the use of fomepizole. Herein, we report a case of acute renal failure caused by ethylene glycol poisoning that was treated with fomepizole and hemodialysis and present a literature review.
Ethylene glycol is a colorless, odorless, and water-soluble substance. It is currently used as a raw material in solvents, antifreezes, diluents, and various chemicals1). It is referred to as a “sweet killer” in the West because it is sweet and does not irritate the oral mucosa when ingested2). Ethylene glycol poisoning often occurs when an alcoholic person ingests the liquid after mistaking it for alcohol, when consumed for the purpose of suicide, or when ingested accidentally by children or elderly persons with cognitive impairment. When ingested, ethylene glycol is quickly absorbed into the body; thus, the mortality rate is high if immediate treatment is not administered3). However, there have not been many cases in Korea. Furthermore, because examination through patient inquiry is often difficult owing to impaired consciousness, diagnosis and treatment may be delayed when the need for a differential diagnosis is not recognized in the absence of an antifreeze bottle found with the patient. Herein, we report the use of fomepizole and hemodialysis to treat a patient with severe high anion gap metabolic and acute renal failure as comorbidities from antifreeze ingestion, along with a literature review.
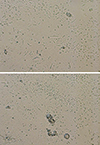
A 28-year-old male patient was admitted to the emergency room with impaired consciousness after ingesting antifreeze for suicide purpose about 5 h earlier. At the time of admission, his guardians brought the container of the antifreeze that he had ingested, which was then checked accordingly. The patient had an elevated blood pressure of 130/79 mmHg, pulse rate of 98 beats/min, and respiration rate of 30 breaths/min at the time of admission; his body temperature was 35.4℃. He showed signs of acute illness and presented with lethargic consciousness and restlessness. No other specific findings were found. The venous blood gas analysis results showed pH 6.90, PaCO2 34 mmHg, PaO2 25 mmHg, bicarbonate 6.7 mmol/L, base excess −26.1 mmol/L, and oxygen saturation 15%. Serum biochemical analysis showed sodium 136.4 mEq/L, potassium 7.80 mEq/L, chloride 110 mEq/L, calcium 9.9 mg/dL, phosphorus 3.2 mg/dL, glucose 125 mg/dL, osmolality 375 mOsmol/kg, blood urea nitrogen (BUN) 11 mg/dL, and creatinine 1.67 mg/dL. The patient also showed signs of high anion gap metabolic acidosis with an anion gap of 19.7 mmol/L and osmolar gap of 91.34 mOsm/kg H20. Urinalysis showed proteinuria and microscopic hematuria. Electrocardiogram findings showed sinus tachycardia with a heart rate of 134 beats/min, whereas chest radiography showed mild pulmonary edema. Fluid replacement, sodium bicarbonate, and thiamine were administered as the initial treatment, and fomepizole was loaded at 15 mg/kg intravenously and administered four times, each at 10 mg/kg in 12-h intervals. One hour after admission, the patient's consciousness deteriorated from lethargy to stupor, necessitating tracheal intubation. Two hours after admission, overall condition deteriorated and acidosis was not corrected. Consequently, he was transferred to the intensive care unit, where continuous renal replacement therapy was initiated. Thirty hours after dialysis, metabolic acidosis was controlled, as indicated by pH 7.46, PaCO2 36 mmHg, PaO2 106 mmHg, HCO3 25.6 mEq/L, and anion gap 5.9 mmol/L (Table 1). His vital signs stabilized on the 6th day of admission, and consequently, he was extubated. However, the patient's levels of BUN and creatinine remained elevated at 39 and 4.28 mg/dL, respectively, and his hourly urine output was only 10–30 mL, and calcium oxalate crystals were detected on urine microscopy (Fig. 1). The patient's acute renal failure persisted, and therefore the treatment was switched to routine hemodialysis, maintained at three rounds every other day. Starting from the 12th day of admission and the start of dialysis, daily urine output increased to >1,000 mL; thus, dialysis was discontinued. On the 23rd day of admission, the serum BUN/creatinine level decreased to 18.4/1.73 mg/dL, and the daily urine output was being maintained at 3000–4000 mL. The patient was discharged and monitored through Follow-ups. During the outpatient follow-up visit at 3 months after discharge, the levels of BUN and creatinine were 17 and 0.95 mg/dL, respectively, with no proteinuria or hematuria (Fig. 2).
Pharmacokinetically, ethylene glycol has a molecular weight of 62 g/mol and is readily soluble in water4). When ingested, it is absorbed within 1–4 h to reach its peak blood concentration. Its elimination half-life is 3 h, whereas the lethal dose has been reported as 1.0–1.5 mL/kg or 100mL in adults5). After its ingestion, about 80% of ethylene glycol is metabolized in the liver, of which 80% is reabsorbed in the proximal tubules and the remaining 20% is excreted in urine4). The absorbed ethylene glycol is converted into four toxic substances by alcohol dehydrogenase: glycoaldehyde, glycolate, glyoxylate, and oxalate6). Ethylene glycol itself has low toxicity; however, symptoms of poisoning may appear due to its metabolites. Particularly, because the process of conversion from glycolate to glyoxylate is slow, glycolate accumulation is the major cause of high anion gap metabolic acidosis and poisoning7). The final product, oxalate, may react with calcium and cause hypocalcemia, in addition to forming calcium oxalate crystals; these crystals get deposited in various tissues such as the brain, heart, liver, vessels, and kidney, thereby causing damage7). Such tissue damage is mostly reversible, and improvement can occur after elimination of ethylene glycol and metabolites and inhibition of metabolite formation. In rare cases, however, the damage may be permanent78).
The clinical progression of ethylene glycol poisoning has been described in three stages. First, the neurologic stage appears between 30min and 12 h after ingestion, during which central nervous system symptoms such as euphoria ataxia, seizure, muscle spasm, and ocular external muscle paralysis mostly appear, and tetany due to hypocalcemia may also appear. Second, the cardiopulmonary stage appears between 12 and 24 h after ingestion, during which symptoms such as tachycardia, hypertension, hyperventilation, congestive heart failure, arrhythmia, and acute respiratory distress syndrome appear. If appropriate treatment is not administered during this period, multiple organ failures may occur, which can lead to death. Third, the renal stage appears between 24 and 72 h after ingestion, during which calcium oxalate crystals are deposited in the tubules, causing acute renal failure69). However, there are significant differences between individuals; in some patients, one stage may be predominant, while other stages may not appear at all. The definitive diagnostic method is to measure the ethylene glycol concentration in the blood; however, it has limitations because most hospitals in Korea are not equipped to conduct such tests10). Therefore, an arterial blood test, an electrolyte test, and urinalysis should be performed in patients suspected of having poisoning or similar symptoms, whereas the anion gap and osmolar gap should be checked in patients presenting with metabolic acidosis. In cases of ethylene glycol poisoning, an elevated osmolar gap and high anion gap metabolic acidosis serve as important clues for the diagnosis. Immediately after poisoning, the osmolar gap is markedly elevated, whereas the anion gap may appear normal. However, as metabolism progresses, the osmolar gap tends to decrease, whereas the anion gap tends to increase11). The concentration of ethylene glycol in the body can be estimated by multiplying the osmolar gap with the conversion factor 6.212). Therefore, in our case, we used the aforementioned method to estimate the concentration of ethylene glycol in the body, giving 566.3 mg/dL, which was considered for the treatment. Additionally, lactic acid value may be elevated in the blood chemistry results, which may be a false-positive result due to structural similarity between glycolic acid and lactate in the lactate oxidase-based test equipment. Equipment that use lactate dehydrogenate do not show such false-positive results, and such difference in the results between two equipment is referred to as a lactate gap13). In our hospital, LX20 (Beckman Coulter, Brea, CA, USA) is used, and the elevated lactic acid level found in the present case may reflect the distressed state of the patient; however, it may also have appeared higher as a false-positive result. Urinalysis results may initially show proteinuria and microscopic hematuria, whereas calcium oxalate crystals may also be found in 50% of cases8). The crystals appear as needleshaped monohydrates and dumbbell-shaped monohydrates, although they may also appear as octahedral envelope-shaped dehydrates when high concentrations of calcium and oxalic acid are present14).
When ethylene glycol is ingested at low concentrations, conservative therapy can be used; however, ingestion of high concentrations may cause serious damage if immediate treatment is not administered. The use of gastric lavage and charcoal is generally known to have little significance owing to the rapid absorption of ethylene glycol. Sodium bicarbonate may be expected to play a role in correcting acidosis and preventing the deprotonation of glycolic and oxalic acids into glycolate and oxalate, respectively, and penetrating into end-organ tissues15). Thiamine and pyridoxine have virtually no adverse effects, and they may be administered as they act as coenzymes in the metabolism of glyoxylate into less toxic substances15). A key treatment after the initial treatment involves fomepizole and ethanol administration; they have a high affinity for alcohol dehydrogenase, and therefore prevent ethylene glycol from being metabolized into toxic substances. Although rare, previously used ethanol may cause hypotension, respiratory failure, hypoglycemia, and hypernatremia; thus, it should be used with caution. Fomepizole is expensive but it has the advantages of not requiring blood concentration monitoring and not causing drunkenness or other adverse effects. Hemodialysis is the most effective method for removing ethylene glycol and metabolites and may be an option when the patient does not respond to appropriate treatments1116).
According to a report by the American Association of Poison Control Centers, 809 cases of ethylene glycol poisoning were reported in 2014, and ethylene glycol poisoning was the third most common cause of poisoning-related deaths after ethanol and carbon monoxide poisoning17). However, the exact number of Korean cases is unknown owing to a lack of statistical data in Korea. In the present case, the diagnosis was made rather easily because the guardians brought the antifreeze container. However, the patient recovered after showing relatively severe progression as he unable to receive immediate appropriate treatment after the poisoning. In conclusion, the possibility of ethylene glycol poisoning should be considered when a patient presents with a significantly elevated osmolar gap, high anion gap metabolic acidosis, impaired consciousness, and renal dysfunction. Furthermore, minimizing the toxic effects of metabolites through aggressive treatment within a short period will help prevent kidney failure and death.
Figures and Tables
References
3. Fraser AD. Clinical toxicologic implications of ethylene glycol and glycolic acid poisoning. Ther Drug Monit. 2002; 24:232–238.

4. Jammalamadaka D, Raissi S. Ethylene glycol, methanol and isopropyl alcohol intoxication. Am J Med Sci. 2010; 339:276–281.

5. Peterson CD, Collins AJ, Himes JM, Bullock ML, Keane WF. Ethylene glycol poisoning: pharmacokinetics during therapy with ethanol and hemodialysis. New Engl J Med. 1981; 304:21–23.
6. Gardner TB, Manning HL, Beelen AP, Cimis RJ, Cates JM, Lewis LD. Ethylene glycol toxicity associated with ischemia, perforation, and colonic oxalate crystal deposition. J Clin Gastroenterol. 2004; 38:435–439.

7. Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002; 40:415–446.

8. Bae JO, Kang SG, Lim SM, et al. A Case of Ethylene Glycol Poisoning with Metabolic Acidosis Treated with Hemodialysis. Kidney Res Clin Pract. 2005; 24:1039–1043.
9. Rahman SS, Kadakia S, Balsam L, Rubinstein S. Autonomic Dysfunction as a Delayed Sequelae of Acute Ethylene Glycol Ingestion. J Med Toxicol. 2012; 8:124–129.

10. Min JH, Lee JY, Min MG, Chung SP, Kim SW, Yoo IS. Treatment of Ethylene Glycol Poisoning Patient Presented with Mental Change. J Korean Soc Clin Toxicol. 2004; 2:129–132.
11. Porter WH. Ethylene glycol poisoning: quintessential clinical toxicology; analytical conundrum. Clin Chim Acta. 2012; 413:365–377.

12. Glaser DS. Utility of the serum osmol gap in the diagnosis of methanol or ethylene glycol ingestion. Ann Emerg Med. 1996; 27:343–346.

13. Meng QH, Adeli K, Zello GA, Porter WH, Krahn J. Elevated lactate in ethylene glycol poisoning: True or false. Clin Chim Acta. 2010; 411:601–604.

14. Hanouneh M, Chen TK. Calcium Oxalate Crystals in Ethylene Glycol Toxicity. New Engl J Med. 2017; 377:1467–1451.

15. Caravati EM, Erdman AR, Christianson G, et al. Ethylene glycol exposure: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2005; 43:327–345.

16. Mégarbane B. Treatment of patients with ethylene glycol or methanol poisoning: focus on fomepizole. Open Access emergency medicine. Open Access Emerg Med. 2010; 2:67–75.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download