This article has been
cited by other articles in ScienceCentral.
Abstract
Posterior reversible encephalopathy syndrome (PRES) is characterized by a clinical and radiological entity with the sudden onset of seizures, headache, altered consciousness, and visual disturbances in patients with the findings of reversible vasogenic subcortical edema without infarction. Hypertension, renal disease, and autoimmune disease are co-morbid conditions of PRES. Nevertheless, there have only been a few case reports of PRES in a patient with anti-glomerular basement membrane antibody glomerulonephritis (anti-GBM GN). This paper presents the possible first Korean case of a 36-year-old woman with the striking features of PRES. She presented with a sudden onset of visual blindness, headache, and seizure. The brain MRI images revealed hyperintense lesions in both the occipital and parietal lobes, which suggested vasogenic edema. Three months before this presentation, she was diagnosed with anti-GBM GN. Since then, she underwent immunosuppression with cyclophosphamide and steroid, and hemodialysis for renal failure with a treatment of anti-GBM GN.
Go to :

Keywords: Posterior reversible encephalopathy syndrome, Anti-glomerular basement membrane antibody glomerulonephritis, Hypertension, Cyclophosphamide
Introduction
Posterior reversible encephalopathy syndrome (PRES), a recently described syndrome that is also referred to as reversible posterior leukoencephalopathy syndrome (RPLS), presents as the sudden onset of seizure, headache, and visual loss. PRES is diagnosed by the characteristic neuroimaging features of reversible vasogenic subcortical edema by cerebral magnetic resonance imaging (MRI)
1). Acute kidney injury and chronic kidney disease commonly present in patients with PRES, and PRES is strongly associated with hypertension, vascular and autoimmune diseases, exposure to immunosuppressive drugs and organ transplantation. PRES associated with diverse connective disease, including systemic lupus erythematosus (SLE), has been recognized increasingly. Nevertheless, the anti-glomerular basement membrane antibody glomerulonephritis (anti-GBM GN) presenting as PRES has been reported in a few cases
2).
This paper presents the possible first Korean case of PRES in a woman with renal failure secondary to anti-GBM GN without pulmonary hemorrhage.
Go to :

Case Report
A 36-year-old woman, who had an unknown origin of fever and general weakness for a month, was referred to the nephrology department for renal dysfunction. At presentation, her blood pressure (BP) and body temperature was 120/70mmHg and 37.9℃, respectively. On the initial laboratory evaluation, her white blood cell count, hemoglobin, hematocrit, and platelet were 12,170/mm
3, 9.8 g/dL, 29.4%, and 565,000/mm
3, respectively. Urinalysis showed proteinuria 2+ with a protein/creatinine ratio of 1.1 g/g in the first morning urine. Her BUN and serum creatinine were 38.2mg/dL and 4.7mg/dL, respectively. Her ESR and CRP were >140mm/h and 13.75mg/L, respectively. The serologic tests for hepatitis were all negative. The immunologic tests showed all negatives of the anti-nuclear antibody Ab, anti-ds DNA Ab, immune complexes, cryoglobulin, anti-phospholipid Ab, and anti-neutrophil cytoplasmic Ab. The chest X-ray showed no active pulmonary lesions and an ultrasound revealed normal-sized kidneys. Over a few days, the urine output dropped and the plasma creatinine increased rapidly to 6.7mg/dL. On the 5
th day, a high titer (>600U/mL) of anti-GBM Ab was reported. A renal biopsy suggested cellular crescents over 90% of glomerulus (
Fig. 1). Therefore, the patient was diagnosed with anti-GBM GN. She was treated with three 500mg boluses of methylprednisolone, followed by prednisolone at 60mg/day along with 5 sessions of plasmapheresis and oral cyclophosphamide (CYP) at 1mg/kg/day. Prednisolone was tapered to 12mg/day after 8 weeks because the patient did not develop a pulmonary hemorrhage. Despite this, she did not recover her renal function and hemodialysis therapy was started using a jugular venous catheter. The patient's overall health was good, and her blood pressure was well-controlled without any antihypertensives. She was started on maintenance hemodialysis and was discharged home on maintenance dialysis three times per week. She continued on oral CYP (1mg/kg/day) and prednisolone (12mg/day).
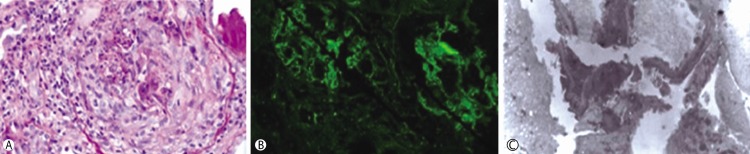 | Fig. 1
(A) Crescentic glomerulus was observed in the renal biopsy and size of glomerulus was markedly enlarged (Periodic acid-Schiff, Hematoxylin-Eosin stain, ×200). (B) Linear infiltration of IgG was shown along the glomerulus basement membrane (GBM). (C) Ruptured GBM was observed by electron microscopy (×5,000).
|
Three months later she presented to the emergency department with a sudden onset of visual blindness and headache. The patient developed two episodes of tonic-clonic seizure that were relieved with benzodiazepines, recovering consciousness between seizures. During the process of remission, we stopped CYP, corticosteroids was maintained (methylprednisolone 12mg/day) (
Fig. 2). Therefore, the possibility that PRES caused by prednisolone was excluded. The BP was 200/120mmHg and the neurological exam showed cortical blindness. The serum electrolytes, glucose, and osmolality were all within the normal levels. The brain MRI on the T2-weighted images showed hyperintense lesions in both occipital and parietal lobes, suggesting vasogenic edema due to the extravasation of fluid, which is also an appropriate finding for PRES (
Fig. 3A). Electroencephalography demonstrated non-specific slow wave changes. The patient was treated with oral phenytoin for seizure and labetalol administered intravenously for BP control. The elevated BP was well controlled by anti-hypertensive medication and the visual acuity returned gradually to normal. She had no further seizures. Repeat MRI2 week later demonstrated a reduction of the mentioned lesions (
Fig. 3B). The seizure drugs were discontinued after 4 weeks.
 | Fig. 2Course of treatment from diagnosis of anti-GBM disease to presentation of neurologic symptoms of PRES.
|
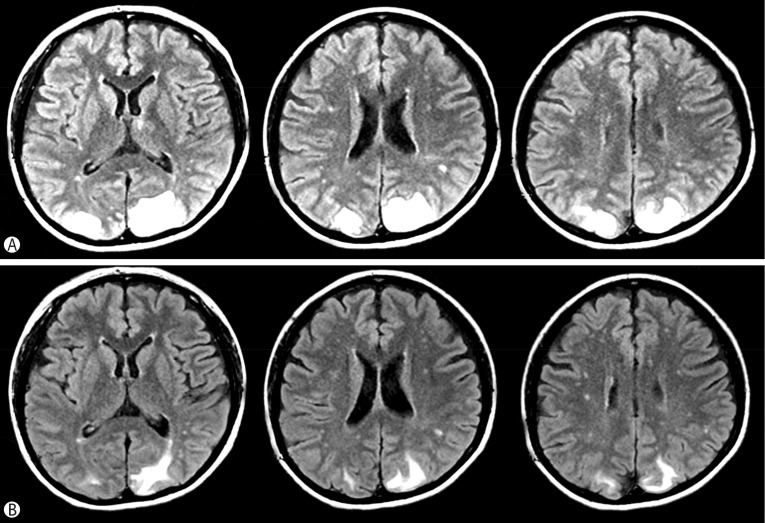 | Fig. 3
Brain MRI Images of the patient.
(A) Several high signals were observed in the bilateral occipital area on the T2-weighted Flair images, suggesting vasogenic edema due to the extravasation of fluid. (B) Reduced T2 high signal were observed in the cortex and subcortex of the parieto-occipital lobes bilaterally on the 14th hospital day.

|
Go to :

Discussion
Anti-GBM GN is an autoimmune disease characterized by rapidly progressive glomerulonephritis (RPGN) and in some cases, is accompanied by pulmonary hemorrhage, also called Goodpasture syndrome. The mortality has been improved by effective treatment with immunosuppressive agents, such as cyclophosphamide and steroid plus plasmapheresis. Unfortunately, renal survival remains very poor because of the delayed diagnosis of anti-GBM GN disease or delayed induction of immunosuppression therapies
3). In this case, upon referral to the hospital, the serum creatinine was already elevated over 4.0mg/dL and the diagnosis of anti-GBM GN with crescents over 90% of glomerulus on the renal biopsy was confirmed. Renal failure did not improve with active immunosuppressive therapy plus plasmapheresis and dialysis. Approximately 12 weeks after discharge, she was readmitted with sudden blindness and seizure with a high blood pressure. She was diagnosed with PRES on the brain MRI T2 images. The abrupt high blood pressure was not believed to be induced by her volume status because she had hemodialysis one day before admission and her recent body weight had not changed.
Clinically, PRES is characterized by visual disturbances, headache, nausea, changes in mental status, and seizure. Seizures are cited as the most common manifestation of PRES, occurring in up to 90% of reported cases. The seizure type is mainly generalized, but focal onset has been reported. Visual disturbances can range from complaints of blurred vision to cortical blindness. Symptoms develop over hours and can persist for weeks, depending on the severity and latency in initiating appropriate care
12). PRES has associations with hypertensive disease conditions, including uncontrolled hypertension, acute and chronic renal failure, and eclampsia. This has also been reported in patients on immunosuppressive and immunomodulatory therapies for malignancy, transplantation, and rheumatologic diseases. Of these, PRES associated with diverse connective diseases including SLE and vasculitis have been reported increasingly. On the other hand, anti-GBM GN presenting PRES have only reported in a few countries (
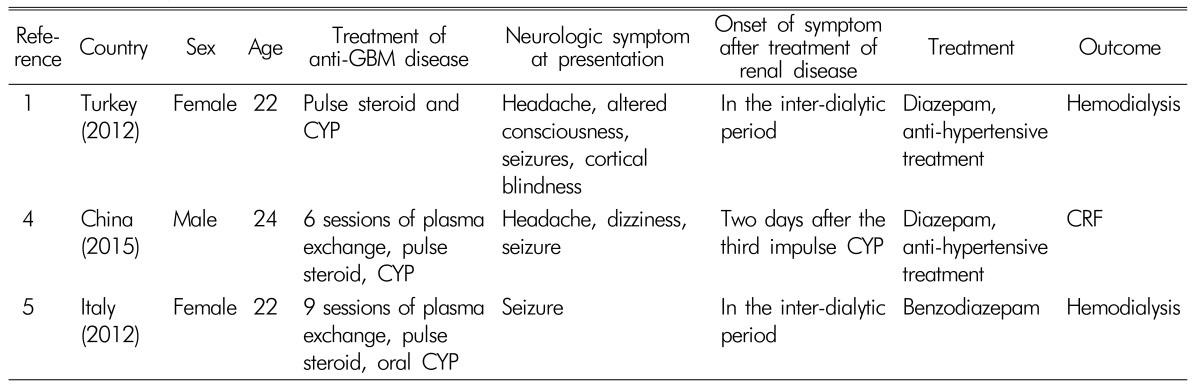
Table 1)
456) and this case is firstly reported in our country. The causes of PRES remain controversial; however, the dysfunction may be caused by a failure of cerebral autoregulation of systemic hypertension or by the cytotoxic effects of vasculitides and by immunosuppressive drugs
7). The present case is unique according to these two hypotheses because PRES developed with a severely elevated blood pressure during the course of immunosuppression using CYP and she received hemodialysis one day before the presence of neurologic symptom with no weight change; thus she was not in volume overload status. Therefore, these two theories for the pathogenesis of vasogenic cerebral edema in PRES appear to be interrelated. In other words, elevated BP disrupts the cerebral autoregulation and autoimmune inflammatory damage plus CYP immu- nosuppressant injury to the endothelium of the cerebral vascular bed
89). In this context, it is very important to control the systolic BP in anti-GBM GN to prevent PRES. Arterial hypertension is detected in approximately 20-30% of patients, so anti-GBM GN alone is susceptible to PRES. Furthermore, some reports have shown that anti-GBM GN in females occurs more frequently during immunosuppression with CYP than in males
10).
Table 1
Reported cases of anti-GBM disease associated with PRES

As in this case, PRES should be suspected in all patients with seizure on maintenance dialysis due to renal failure secondary to autoimmune glomerulonephritis because it is a reversible disease with good control of the BP and short-term anti-seizure medication without any neurological sequelae.
Go to :

Acknowledgments
The authors have no conflicts of interest to declare.
Go to :

References
1. Ozkok A, Elcioglu OC, Bakan A, Atilgan KG, Alisir S, Odabas AR. Reversible posterior leukoencephalopathy in the course of Goodpasture syndrome. Ren Fail. 2012; 34(2):254–256. PMID:
22251235.

2. Camara-Lemarroy CR, Cruz-Moreno MA, Gamboa-Sarquis RN, Gonzalez-Padilla KA, Tamez-Perez HE, Galarza-Delgado DA. Goodpasture syndrome and posterior reversible encephalopathy syndrome. J Neurol Sci. 2015; 354(1-2):135–137. PMID:
25982503.

3. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: Clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015; 14(9):914–925. PMID:
26184985.

4. Ge YT, Liao JL, Liang W, Xiong ZY. Anti-glomerular basement membrane disease combined with IgA nephropathy complicated with reversible posterior leukoencephalopathy syndrome: An unusual case. Am J Case Rep. 2015; 16:849–853. PMID:
26621456.

5. Gutiérrez-Sánchez MJ, Petkov-Stoyanov V, Martín-Navarro JA. Reversible posterior leukoencephalopathy syndrome in Goodpasture syndrome. Nefrologia. 2012; 32(4):540–541. PMID:
22806294.
6. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334(8):494–500. PMID:
8559202.

7. Hirayama K, Yamagata K, Kobayashi M, Koyama A. Anti-glomerular basement membrane antibody disease in Japan: Part of the nationwide rapidly progressive glomerulonephritis survey in Japan. Clin Exp Nephrol. 2008; 12(5):339–347. PMID:
18392773.

8. Jayaweera JL, Withana MR, Dalpatadu CKP, Beligaswatta CD, Rajapakse T, Jayasinghe S, Chang T. Cyclophosphamide-induced posterior reversible encephalopathy syndrome (PRES): a case report. J Med Case Rep. 2014; 8:442. PMID:
25519913.

9. Lee VH, Wijdicks EFM, Manno EM, Rabinstein AA. Clinical Spectrum of Reversible Posterior Leukoencephalopathy Syndrome. 2008; 65(2):205–210.

10. Abenza-Abildua MJ, Blanca F, Domingo D, Aranzazu R, Teresa O, Maria JAA, Exuperio DT. Cyclophosphamide-induced reversible posterior leukoencephalopathy syndrome. BMJ Case Rep. 2009.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download