Abstract
Vitamin D has the pleiotropic effects in multiple organ systems, and vitamin D deficiency was suggested to be associated with high blood pressure according to previous reports. Several interventional studies have examined the effect of vitamin D supplementation on high blood pressure patients, but the results have been inconsistent. In this article, we examined the literature that have proposed a mechanism involving vitamin D in the regulation of blood pressure and review previous observational and interventional studies that have shown the relationship between vitamin D and hypertension among various populations.
Primarily, vitamin D is known as a group of fat-soluble steroids and responsible for prevention of rickets or osteomalacia through increasing intestinal absorption of calcium, iron, magnesium, phosphate, and zinc1). However, there has been a significant improvement in our understanding of the pleiotropic effects of vitamin D in multiple organ systems besides skeletal homeostasis in recent years2). Indeed, receptors for 1, 25-dihydroxyvitamin D, which is the active form of vitamin D have been identified in most human tissues2).
Hypertension, also known as raised blood pressure, is a very common chronic disease and considered as a silent killer because it rarely causes symptoms3). Interestingly, many animal model and observational studies suggested that the vitamin D deficiency closely correlated with cardiovascular disease, especially hypertension456789). Moreover, several interventional studies examined the effect of vitamin D supplementation on high blood pressure in patients although the results were inconsistent4101112131415161718192021222324252627282930313233343536373839404142434445).
Generally, older age, lower incomes and higher body mass index are proposed as the associated factors with the risk of hypertension46). Accordingly, people having high blood pressure would increase in the condition of population ageing and prevalent westernized diet in Korean society46). Therefore, it is very important to look into the evidences and results about vitamin D in regards with its roles in controlling blood pressure at this point.
In this article, we examine the literatures that proposed mechanism of vitamin D on the regulation of blood pressure and review previous observational and interventional studies showing the relationship between vitamin D and hypertension among various populations.
There are two important compounds among the vitamin D groups in humans, which are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol)1). Cholecalciferol and ergocalciferol are found in few types of foods, so sunlight exposure is the main source of vitamin D for humans, other than supplements124748). Solar ultraviolet B radiation (wavelength, 290 to 315nm) penetrates the skin and converts 7-dehydrocholesterol to previtamin D3, which is spontaneously isomerized into vitamin D3 (cholecalciferol). Vitamin D from photosynthesis or food ingestion is metabolized in the liver to 25-hydroxyvitamin and subsequently hydroxylated in the renal proximal tubules by the enzyme 1α-hydroxylase to 1, 25-dihydroxy-vitamin D(1,25 (OH)2D, calcitriol), the biologically active form. 1,25 (OH)2D promotes intestinal calcium absorption2). When vitamin D deficiency decreases the absorption of dietary calcium and phosphorus, the level of parathyroid hormone (PTH) increases2495051). Sufficient vitamin D stimulates calcium and phosphorus absorption by 30-40% and 80% respectively. Without vitamin D, no more than 10-15% of dietary calcium and approximately 60% of phosphorus are absorbed4952).
Vitamin D-Binding proteins (DBP) are synthesized in hepatocytes and helps vitamin D to transport to target organs. Because DBP is the primary transporter of vitamin D and its metabolites, it has a role in maintaining the total levels of vitamin D for the organism and in regulating the amounts of free vitamin D available for specific tissues and cell types to utilize53). DBP linked vitamin D is actively transported by megalin mediated endocytosis in the various target cells54), and intracellular vitamin D binding proteins (IDBPs) help to regulate the intracellular metabolism of vitamin D thereafter55).
The renal production of 1,25 (OH)2D is tightly regulated by 1, 25-dihydroxyvitamin D itself, plasma PTH levels as a signal of calcium homeostasis, and fibroblast growth factor 23 (FGF 23) as a signal of phosphate status5657). Free 1,25 (OH)2D can form a complex with vitamin D receptor, the VDR, and reduce transcription of CYP27B1 (1α-hydroxylase)58). PTH is a hormone secreted by the parathyroid glands which regulates serum calcium through its effects on bone, kidney, and the intestine59). When the level of serum calcium decrease, the production of PTH in parathyroid gland increase and lead to calcium resorption from bone and the renal tubular fluid6061). In addition, PTH up-regulates 1α-hydroxylase enzyme, which converts inactive vitamin D into 1,25 (OH)2D60). FGF 23 is secreted by osteocytes in response to elevated 1,25 (OH) 2D and increased plasma levels of phosphorous62). FGF23 has three types of effect. First, FGF23 impairs sodiumphosphate cotransporters on the kidneys and small intestines, through internalization of the transporters by the cells and consequently, phosphate loss occurs62). FGF23 also inhibits production of 1,25 (OH)2D and promotes breakdown of 1,25 (OH)2D257). Lastly, FGF 23 Inhibits production and secretion of parathyroid62). All three roles of FGF 23 contribute to decrease lowering the level of serum phosphate.
Vitamin D receptor (VDR) is present in thirty-six tissues and also at least 10 tissues possess 1α-hydroxlyase besides the renal proximal tubule47). These facts mean the cells in various tissues need vitamin D during their biological actions63). Several mechanisms have been suggested to be involved in the pathogenesis of hypertension6465).
Inappropriate activation of renin-angiotensin-aldosterone system(RAAS) has been widely known as the important factor contributing to the development of hypertension66). For that reason, blockers of the RAAS, such as renin inhibitors, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor antagonists, and mineralocorticoid receptor antagonist are very important drugs in the treatment of hypertension66). Surprisingly, vitamin D, the sunshine hormone was shown to regulate the RAAS at the clinical, pathophysiological and molecular levels in animals and human studies6768).
The first epidemiological studies proposed an inverse correlation between vitamin D and renin levels were published more than two decades ago69). Recently, this relationship was confirmed in 184 normotensive individuals by Forman et al.67). They reported that the individuals with suboptimal vitamin D levels had higher circulating angiotensin II levels and blunted renal plasma flow responses to infused angiotensin II suggesting activation of the RAAS in the setting of lower plasma 25 (OH)D67). Li et al. documented that absence of vitamin D signaling caused an increase in renin gene expression and plasma angiotensin II in mice lacking the VDR70). Consequently, VDR null mice showed high blood pressure, cardiac hypertrophy and increased water intake70). Likewise, other animal study using mice lacking 1-α hydroxylase showed similar phenotypes with VDR null mice71). That suppressive action of vitamin D on renin was independent of extracellular calcium or phosphorus7071). Mechanistic study using the mouse Ren-1c gene promoter indicated that 1,25(OH)2D3 binds to the VDR, and subsequently liganded VDR blocks formation of the cAMP-response element-binding protein complexes in the promoter region of the renin gene, leading to down-regulation of gene expression72).
Immunologic basis is also mediated with common hypertension and recent studies have strengthened the concept737475). In the experimental studies using mice which lack T or B cells, the degree of hypertension induced by angiotensin II or norepinephrine infusion was markedly blunted7475). Moreover, cytokines released from T cells and other inflammatory cells may be connected with the development of prominent hypertension73). Actually, etanercept, the TNFα antagonist has been shown to have effects on relieving hypertension in the mice fed on fructose76). Interleukins such as IL-6, IL-17, and IL 10 were also reported as involved in hypertension in several studies777879). Intriguingly, immune cells including macrophages, T and B cells are known to have VDRs and several studies have confirmed that vitamin D plays a crucial role in modulating innate and adaptive immune response in various disease4780). Vitamin D acts to modulate toll like receptor (TLR) signaling, which results in reduces the gene expression and protein release of proinflammatory mediators, such as TNFα, IL-6, and MCP-180). From these perspectives, it sounds plausible that anti-inflammatory effects of vitamin D could mitigate the development of hypertension.
Endothelial cells were reported to contain VDRs, and vitamin D supplement has been shown to improve endothelial functions in previous reports1581). Sugden JA et al. examined whether vitamin D2 can improve endothelial function in type 2 diabetes patients with low serum 25(OH)D level15). They found that a single dose supplement of 100,000U ergocalciferol (vitamin D2) significantly improved flow mediated vasodilation (FMD) of the brachial artery by 2.3% and decreased systolic blood pressure by 14mmHg compared with placebo15). In another study, FMD measurements were significantly lower in the asymptomatic people with vitamin D deficiency but it increased after three month-replacement of vitamin D381). The patients with chronic kidney disease (CKD) are reported to have a high prevalence of vitamin D deficiency due to loss of vitamin D binding proteins in the urine, ineffective vitamin D photosynthesis in the skin, low level of 1α-hydroxylase activity, elevated FGF23 levels, and malnutrition etc.8283). Several observational studies identified positive relationship between the level of 25(OH)D and FMD in CKD patients8485). Additionally, animal study found that absence of vitamin D activities caused reduced expression of endothelial nitric oxide synthase resulting in increased arterial stiffness86).
Although lots of interventional studies have been conducted to date, the effect of vitamin D supplement on blood pressure lowering in human is still controversial4101112131415161718192021222324252627282930313233343536373839404142434445) (Table 1).
Lind L et al. showed reduction of diastolic blood pressure by treatment of 1 µg alphacalcidol, a synthetic analogue of active vitamin D during a six-month, double-blind, placebo-controlled trials in the subjects with hypercalcemia (n=29) or primary hyperparathyroidism(n=33) in the late 1900s1011). However, they failed to prove the effect of alphacalcidol on hypertension in the subjects with impaired glucose tolerance (n=16) around the same time87). In 1998, Krause R et al. reported that short term ultraviolet B exposure had an effect on blood pressure lowering in patients with untreated mild hypertension with increase of plasma 25(OH)D concentration88).
In several studies, a single high dose of active vitamin D(100,000 IU) showed improvement in SBP among type 2 diabetes patients1521). On the other hands, the highest single dose of vitamin D3 (300,000 IU) did not reduce blood pressure in the patients with type 2 diabetes25). Daily supplementation of vitamin D3 (1,000-5,000 IU) have failed to prove the effect on blood pressure313942). However, Sharb-Bidar S et al. reported that combination of vitamin D3 plus calcium supplementation had an effect on SBP and DBP in 100 patients with type 2 diabetes22).
Eight week vitamin D3 plus calcium improved blood pressure in elderly women14). However, the Women's Health Initiative Calcium/Vitamin D Trial16) which was a randomized double blind fashion and conducted in 36,282 postmenopausal women showed vitamin D3 (400 IU) plus calcium(1,000mg) daily supplementation was ineffective on either decrease of blood pressure or the risk of developing hypertension over a median follow-up time of 7 years.
It is possible that vitamin D could lower BP in other specific race/ethnic groups. Skin color is a factor of circulating levels of 25(OH)D, and African-Americans are generally known to have significantly higher rates of hypertension than whites33). Forman JP reported that SBP was reduced after the supplementation of vitamin D3 for 3 months compared to placebo in normotensive African-Americans33). Interestingly, the more SBP lowering was observed in the individuals with low vitamin D levels (<20 nM/mL). In comparison, Scragg R et al. found no effects of high dose vitamin D3 during 18 months on blood pressure control in healthy adults who were predominantly Whites41). However, other short-term studies of nonwhite populations failed to prove the effect of vitamin D on BP, although this could be a result of their low statistical power because of their small sample sizes (n≤100)178990).
Recently, several meta-analysis were reported about the effect of vitamin D supplementation on hypertension646591). In a meta-analysis by Wu including four double-blind randomized controlled trials (RCTs) of oral vitamin D supplementation in normotensive or hypertensive individuals (429 participants), vitamin D significantly decreased SBP by 2.44mmHg but not DBP91). They also suggested that the change of blood pressure was not influenced due to the dose of vitamin D supplementation, study length, or additional calcium supplementation in subgroup analysis91). However, other meta-analyses indicated that vitamin D supplementation was not beneficial for blood pressure control646592). Kunter SK et al. examined 16 RCTs of oral vitamin D (vitamin D2 or D3) and concluded that there was no significant effect of vitamin D on blood pressure lowering92). Meanwhile, subgroup analysis showed significant reduction in DBP among the participants with cardiometabolic disease87). Beveridge KA et al. included 46 RCTs (4,541 participants) in the trial-level meta-analysis and individual data from 27 RCTs (3,092 participants) that used active or inactive forms of vitamin D or vitamin D analogues for more than 4 weeks64). No effect of vitamin D treatment on BP was observed at the trial level and individual data64). Total 917 individuals from eight RCTs using active vitamin D for more than 3 months were analyzed by Qi D et al.65). Their meta-analysis found a slight but not significant reduction on blood pressure65).
To date, the results of RCTs and meta-analysis of them do not support the use of vitamin D or its analogues as an individual patient treatment for hypertension or as a population-level intervention to lower BP. Those discrepancies might be due to heterogeneity of patient baseline characteristics, differences in sample size and follow-up periods, and different vitamin D doses. However, many experimental and epidemiologic studies showed possible roles of vitamin D in controlling BP in various ways6465). Further RCTs are required to confirm the real effect of vitamin D on blood pressure reduction and define the optimum dose, dosing interval, and type of vitamin D to administer.
References
1. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006; 81:353–373. PMID: 16529140.

3. Lackland DT, Weber MA. Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol. 2015; 31:569–571. PMID: 25795106.

4. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007; 49:1063–1069. PMID: 17372031.

5. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008; 117:503–511. PMID: 18180395.

6. Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008; 52:828–832. PMID: 18838623.

7. Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008; 87:136–141. PMID: 18175747.

8. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007; 20:713–719. PMID: 17586404.

9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014; 27:114–121. PMID: 24042165.

10. Lind L, Wengle B, Ljunghall S. Blood pressure is lowered by vitamin D (alphacalcidol) during long-term treatment of patients with intermittent hypercalcaemia. A double-blind, placebo-controlled study. Acta Med Scand. 1987; 222:423–427. PMID: 3321926.
11. Lind L, Wengle B, Wide L, Sorensen OH, Ljunghall S. Hypertension in primary hyperparathyroidism--reduction of blood pressure by long-term treatment with vitamin D(alphacalcidol). A double-blind, placebo-controlled study. Am J Hypertens. 1988; 1:397–402. PMID: 3063290.
12. Lind L, Wengle B, Wide L, Ljunghall S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am J Hypertens. 1989; 2:20–25. PMID: 2643969.
13. Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995; 49:640–646. PMID: 7498100.
14. Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001; 86:1633–1637. PMID: 11297596.

15. Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008; 25:320–325. PMID: 18279409.
16. Margolis KL, Ray RM, Van Horn L, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension. 2008; 52:847–855. PMID: 18824662.
17. Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009; 26:19–27. PMID: 19125756.
18. Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009; 89:1321–1327. PMID: 19321573.

19. de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010; 376:1543–1551. PMID: 21055801.

20. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010; 3:195–201. PMID: 20103775.

21. Witham MD, Dove FJ, Dryburgh M, et al. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010; 53:2112–2119. PMID: 20596692.

22. Shab-Bidar S, Neyestani TR, Djazayery A, et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med. 2011; 9:125. PMID: 22114787.

23. Alvarez JA, Law J, Coakley KE, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012; 96:672–679. PMID: 22854402.

24. Gepner AD, Ramamurthy R, Krueger DC, et al. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012; 7:e36617. PMID: 22586483.

25. Heshmat R, Tabatabaei-Malazy O, Abbaszadeh-Ahranjani S, et al. Effect of vitamin D on insulin resistance and anthropometric parameters in Type 2 diabetes; a randomized double-blind clinical trial. Daru. 2012; 20:10. PMID: 23351271.

26. Muldowney S, Lucey AJ, Hill TR, et al. Incremental cholecalciferol supplementation up to 15mug/d throughout winter at 51-55 degrees N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. J Nutr. 2012; 142:1519–1525. PMID: 22739371.
27. Salehpour A, Shidfar F, Hosseinpanah F, et al. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. Br J Nutr. 2012; 108:1866–1873. PMID: 22317756.

28. Stricker H, Tosi Bianda F, Guidicelli-Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high-dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg. 2012; 44:307–312. PMID: 22831874.

29. Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012; 22:864–870. PMID: 21194910.

30. Wood AD, Secombes KR, Thies F, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012; 97:3557–3568. PMID: 22865902.
31. Breslavsky A, Frand J, Matas Z, et al. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. 2013; 32:970–975. PMID: 23561637.

32. Chai W, Cooney RV, Franke AA, Bostick RM. Effects of calcium and vitamin D supplementation on blood pressure and serum lipids and carotenoids: a randomized, double-blind, placebo-controlled, clinical trial. Ann Epidemiol. 2013; 23:564–570. PMID: 23958407.
33. Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013; 61:779–785. PMID: 23487599.

34. Larsen T, Mose FH, Bech JN, Pedersen EB. Effect of paricalcitol on renin and albuminuria in non-diabetic stage III-IV chronic kidney disease: a randomized placebo-controlled trial. BMC Nephrol. 2013; 14:163. PMID: 23889806.

35. Toxqui L, Blanco-Rojo R, Wright I, Perez-Granados AM, Vaquero MP. Changes in blood pressure and lipid levels in young women consuming a vitamin D-fortified skimmed milk: a randomised controlled trial. Nutrients. 2013; 5:4966–4977. PMID: 24317556.
36. Wamberg L, Kampmann U, Stodkilde-Jorgensen H, et al. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013; 24:644–649. PMID: 23566943.

37. Witham MD, Adams F, Kabir G, et al. Effect of shortterm vitamin D supplementation on markers of vascular health in South Asian women living in the UK--a randomised controlled trial. Atherosclerosis. 2013; 230:293–299. PMID: 24075759.
38. Witham MD, Dove FJ, Khan F, et al. Effects of vitamin D supplementation on markers of vascular function after myocardial infarction--a randomised controlled trial. Int J Cardiol. 2013; 167:745–749. PMID: 22459388.

39. Yiu YF, Yiu KH, Siu CW, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013; 227:140–146. PMID: 23298824.

40. Dalbeni A, Scaturro G, Degan M, Minuz P, Delva P. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Nutr Metab Cardiovasc Dis. 2014; 24:861–868. PMID: 24787908.

41. Scragg R, Slow S, Stewart AW, et al. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults: a randomized controlled trial. Hypertension. 2014; 64:725–730. PMID: 24980662.
42. Strobel F, Reusch J, Penna-Martinez M, et al. Effect of a randomised controlled vitamin D trial on insulin resistance and glucose metabolism in patients with type 2 diabetes mellitus. Horm Metab Res. 2014; 46:54–58. PMID: 24198221.

43. Sollid ST, Hutchinson MY, Fuskevag OM, et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014; 37:2123–2131. PMID: 24947792.

44. Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014; 25:175–186. PMID: 24052631.

45. Witham MD, Ireland S, Houston JG, et al. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: randomized, controlled trial. Hypertension. 2014; 63:706–712. PMID: 24420547.
46. Jo I, Ahn Y, Lee J, et al. Prevalence, awareness, treatment, control and risk factors of hypertension in Korea: the Ansan study. J Hypertens. 2001; 19:1523–1532. PMID: 11564970.

47. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008; 88:491s–499s. PMID: 18689389.

48. Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005; 135:310–316. PMID: 15671233.

49. Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006; 260:245–254. PMID: 16918822.

50. Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004; 80:1706s–1709s. PMID: 15585791.

51. Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005; 90:3215–3224. PMID: 15797954.

52. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007; 85:1586–1591. PMID: 17556697.

53. Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012; 30:445–456. PMID: 22528806.

54. Cooke NE, Haddad JG. Vitamin D binding protein (Gcglobulin). Endocr Rev. 1989; 10:294–307. PMID: 2476303.

55. Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973; 154:566–574. PMID: 4691503.

56. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006; 116:2062–2072. PMID: 16886050.

57. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004; 80:1689s–1696s. PMID: 15585789.

58. Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011; 25:531–541. PMID: 21872796.

59. Coetzee M, Kruger MC. Osteoprotegerin-receptor activator of nuclear factor-kappaB ligand ratio: a new approach to osteoporosis treatment? South Med J. 2004; 97:506–511. PMID: 15180028.
60. Poole KE, Reeve J. Parathyroid hormone - a bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005; 5:612–617. PMID: 16181808.

61. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015; 10:1257–1272. PMID: 25287933.

62. Juppner H. Phosphate and FGF-23. Kidney Int Suppl. 2011; S24–S27. PMID: 21346724.
63. Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012; 3:118–126. PMID: 22629085.
64. Beveridge LA, Struthers AD, Khan F, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015; 175:745–754. PMID: 25775274.
65. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: A systemic review and meta-analysis. Int J Cardiol. 2017; 227:177–186. PMID: 27866065.

66. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015; 116:960–975. PMID: 25767283.
67. Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010; 55:1283–1288. PMID: 20351344.

68. Santoro D, Caccamo D, Lucisano S, et al. Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. Biomed Res Int. 2015; 2015:145828. PMID: 26000281.

69. Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990; 3:903–905. PMID: 2081010.

70. Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002; 110:229–238. PMID: 12122115.

71. Zhou C, Lu F, Cao K, et al. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1α-hydroxylase knockout mice. Kidney Int. 2008; 74:170–179. PMID: 18385669.

72. Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007; 282:29821–29830. PMID: 17690094.

73. Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011; 57:132–140. PMID: 21149826.

74. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007; 204:2449–2460. PMID: 17875676.
75. Crowley SD, Song YS, Lin EE, et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R1089–R1097. PMID: 20147609.

76. Tran LT, MacLeod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem. 2009; 330:219–228. PMID: 19440659.

77. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007; 27:2576–2581. PMID: 17962626.
78. Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007; 179:4135–4141. PMID: 17785852.

79. Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006; 212:8–27. PMID: 16903903.
80. Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014; 7:69–87. PMID: 24971027.
81. Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009; 94:4023–4030. PMID: 19584181.

82. Lee SY, Kim HY, Gu SW, Kim HJ, Yang DH. 25-hydroxyvitamin D levels and vascular calcification in predialysis and dialysis patients with chronic kidney disease. Kidney Blood Press Res. 2012; 35:349–354. PMID: 22487876.

83. Ko EJ, Kim BH, Jeong HY, et al. Serum 25-hydroxyvitamin D as a predictor of hospitalization-free survival in predialysis and dialysis patients with chronic kidney disease: a single-center prospective observational analysis. Kidney Res Clin Pract. 2016; 35:22–28. PMID: 27069854.

84. Chitalia N, Recio-Mayoral A, Kaski JC, Banerjee D. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis. 2012; 220:265–268. PMID: 22071357.

85. Zhang QY, Jiang CM, Sun C, et al. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J Nephrol. 2015; 28:471–476. PMID: 25515034.

86. Andrukhova O, Slavic S, Zeitz U, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014; 28:53–64. PMID: 24284821.

87. Lind L, Pollare T, Hvarfner A, et al. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res. 1989; 11:141–147. PMID: 2697485.
88. Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998; 352:709–710.

89. Pan WH, Wang CY, Li LA, Kao LS, Yeh SH. No significant effect of calcium and vitamin D supplementation on blood pressure and calcium metabolism in elderly Chinese. Chin J Physiol. 1993; 36:85–94. PMID: 8198625.
90. Salehpour A, Hosseinpanah F, Shidfar F, et al. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012; 11:78. PMID: 22998754.

91. Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010; 103:729–737. PMID: 20622727.

92. Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014; 29:1–14. PMID: 24374742.

93. Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010; 267:462–472. PMID: 20141565.
94. Witham MD, Price RJ, Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013; 173:1672–1679. PMID: 23939263.
Table 1
Randomized controlled trials concerning the effects of vitamin D or vitamin D analogue supplementation on blood pressure
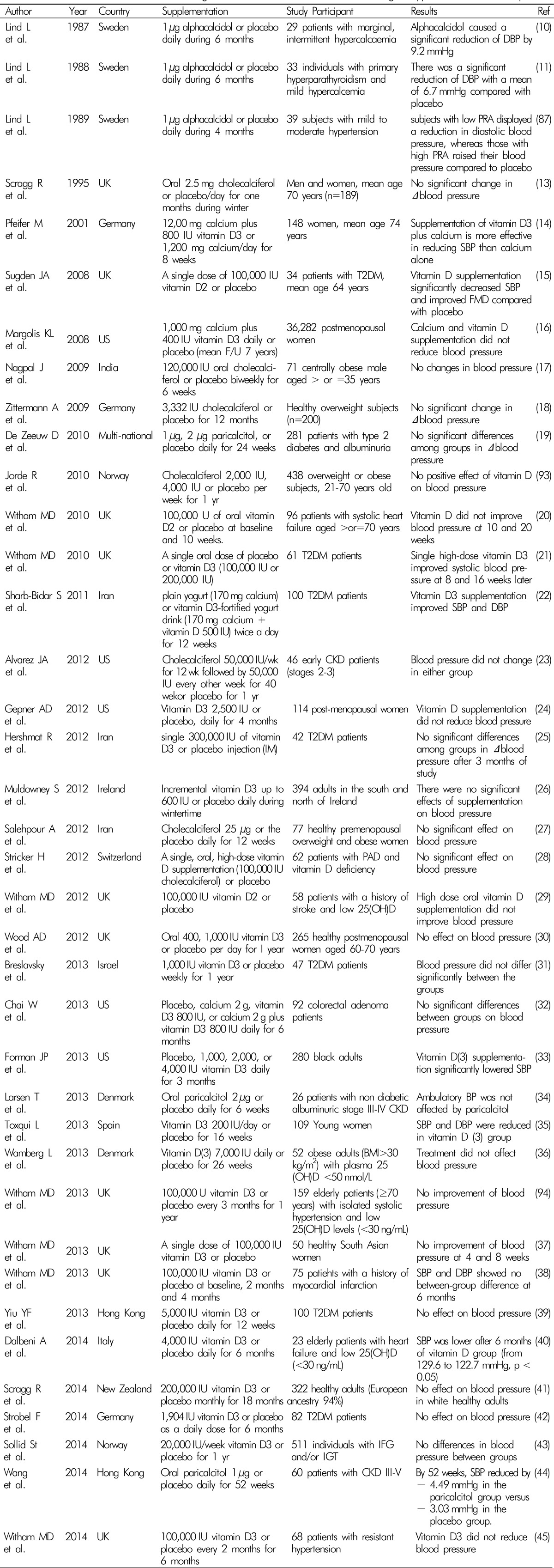
Abbreviations: CKD, chronic kidney disease; DBP, diastolic blood pressure; FMD, flow mediated dilatation; GDM, gestational diabetic mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; PAD, peripheral artery disease; PRA, plasma renin activity; RCT, randomized controlled trial; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download