Abstract
The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is the most common cause of euvolemic hyponatremia, and many medications have been associated with SIADH. Pregabalin is a drug used for the treatment of neuropathic pain, though common adverse effects include central nervous system disturbance, peripheral edema, and weight gain. However, hyponatremia caused by pregabalin has been rarely reported. Here we report a patient with pregabalin-induced hyponatremia who met the criteria for SIADH; after discontinuation of the drug, his condition rapidly improved. This case can help clinicians diagnose and treat new-onset hyponatremia in patients who recently initiated pregabalin therapy.
Go to : 
Hyponatremia is the most frequent electrolyte disturbance among hospitalized patients12). In addition, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) is the most common cause of euvolemic hyponatremia3). SIADH is characterized by either the sustained release of antidiuretic hormone (ADH) in the absence of stimuli, or by the enhanced action of ADH on the kidney4). Causes of SIADH include malignant disease, pulmonary disorders, central nervous system disorders, and a number of medications1). Several drugs are known to cause this syndrome; however, there are only few reported cases in which SIADH was induced by pregabalin. Therefore, here we report a patient with pregabalin-induced SIADH.
Go to : 
A 69-year-old man presented with general weakness and fever. A chest computed tomography (CT) revealed consolidation in both lower lobes of the lungs, and he was diagnosed with pneumonia. The patient had been treated with numerous drugs, including an angiotensin-converting enzyme inhibitor, metformin, a DPP-IV inhibitor, and thioctacid, for management of type 2 diabetes mellitus and hypertension. Several weeks prior to hospital admission, he had a severe ulcer on his right heel caused by arteriosclerosis obliterans. Shortly before admission, he initiated pregabalin therapy to relieve the pain of the right heel.
On admission, the patient's blood pressure was 128/58 mmHg, and his pulse rate was 76 beats per minute. His body temperature was 37.8℃, and crackles were heard on both sides of the lung field. Physical examination did not reveal any neurologic dysfunctions or other symptoms, except mild dyspnea. Hematological analysis demonstrated a white blood cell count of 11,150/mm3 (reference range, 400-10,000/mm3), and a hemoglobin level of 9.6 g/dL (reference range, 13-17.5 g/dL). Biochemical analysis demonstrated a sodium level of 138 mEq/L (reference range, 136-145 mEq/L) and a potassium level of 5.0 mEq/L (reference range, 3.5-5.1 mEq/L). There was no abnormality in the results of the liver function tests or renal function tests. As previously stated, the patient was diagnosed with pneumonia; thus, we initiated administration of ceftriaxone and clarithromycin to treat the pneumonia. The patient's condition improved, and antibiotic therapy was ceased after 2 weeks of administration.
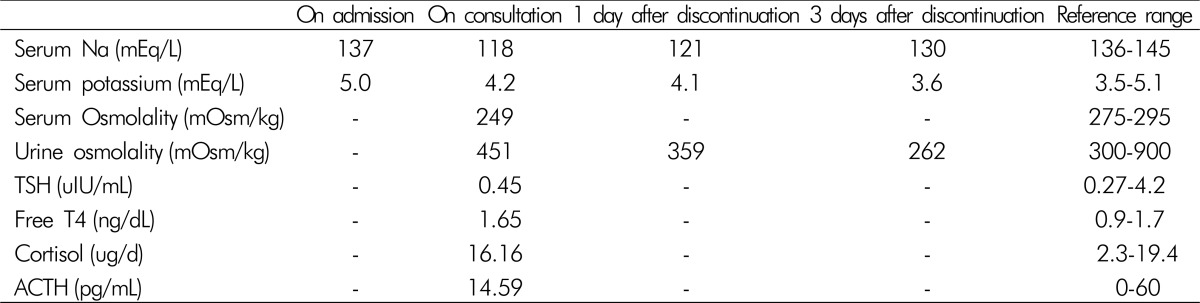
At the point of termination of pneumonia treatment, the patient was found to be hyponatremic, with a sodium level of 122 mEq/L. His serum sodium level further decreased to 118 mEq/L. Chest radiography revealed an improvement in pneumonia. Additional endocrinologic studies that evaluated hyponatremia excluded hypothyroidism and adrenal insufficiency. Laboratory studies revealed a reduced serum osmolality (249 mOsm/kg). Additionally, the patient's random spot urine sodium and osmolality levels were 91 mEq/L (reference range, 40-220 mEq/L), and 451 mOsm/kg (reference range, 300-900 mOsm/kg), respectively. Based on the patient's clinical euvolemia and the biochemical data, SIADH was diagnosed. The patient received no other medications, except antibiotics for pneumonia treatment. He was then placed on fluid restriction, but it was unsuccessful for correction of the serum sodium level. Subsequently, 3% saline was supplied; however, laboratory findings indicated a serum sodium level of 116 mEq/L. Therefore, he was referred to the nephrology clinic for the treatment and evaluation of SIADH.
The endocrinologic tests and his medications were reviewed. Tests for thyroid and adrenal function did not show any abnormalities. Therefore, we presumed that SIADH might be induced by pregabalin, and decided to stop its administration. The serum sodium and urine osmolality were again evaluated, followed by administration of 0.9% isotonic saline. After the pregabalin discontinuance, the serum sodium level increased to 121 mEq/L with a decreased urine osmolality of 389 mEq/L. Two days later, the serum sodium level of the patient increased to a near-normal level of 130 mEq/L (Table 1). To determine whether an adverse drug reaction was caused by pregabalin, we used the Naranjo algorithm(Table 2). The total score was 6, which equates to “probable” as the probability that an adverse event was related to the drug therapy. The patient remained asymptomatic, even when his sodium level was decreased to 116 mEq/L. His biochemical findings improved after stopping pregabalin therapy without any side effects.
Go to : 
Drug-induced SIADH can occur owing to an increased sensitivity to ADH in the nephron or an increase in ADH production4). Increased ADH activity impairs the ability of the kidney to dilute urine, resulting in decreased excretion of ingested water and a highly concentrated and decreased urine volume56). Patients with SIADH present with a euvolemic status because the excess water distributes evenly throughout the body's fluid compartments5). Selective serotonin reuptake inhibitors (SSRIs), various chemotherapeutic agents (e.g., cyclophosphamide, cisplatin, and vinca alkaloids), carbamazepine, and antipsychotics appear to be most strongly associated with drug-induced SIADH56).
Pregabalin is an analog of the neurotransmitter gamma-aminobutyric acid (GABA) that has analgesic, anticonvulsant, and anxiolytic properties7). It is now widely used in the management of diabetic peripheral neuropathy, post-herpetic neuralgia, generalized anxiety disorder, and social anxiety disorder. The most common adverse events associated with pregabalin include dizziness and somnolence, followed by peripheral edema and weight gain8). Hyponatremia is an uncommon side effect of pregabalin.
Several articles have reported decompensation of patients with chronic heart failure, edema, and weight gain, all caused by pregabalin78). The decompensated heart failure was resolved after discontinuation of the drug. Although the mechanism of pregabalin is not well known, the calcium channel relationship may cause these side effects. Pregabalin is an alpha2-delta ligand that has analgesic, anticonvulsant, and anxiolytic activity. Alpha2-delta is an auxiliary protein associated with voltage-gated calcium channels. They potently bind to the subunit, resulting in modulation of calcium channels and reduction in the release of several neurotransmitters9). The effect of alpha2-delta type 1 and 2 subunits on calcium channel functions and conventional calcium channel subtypes are not clear10). This calcium channel relationship, however, may lead to peripheral edema with peripheral vasodilation and fluid leakage into the interstitial space. It is thought that unexplained weight gain may be a sign of fluid retention, which may exacerbate congestive heart failure11). In addition, it is inferred that SIADH can develop as a result of this fluid leakage into the interstitial space and fluid retention.
Although a large number of drugs are known to potentially induce SIADH, pregabalin-induced SIADH has rarely been reported. Only two cases have been reported previously. In one case, a patient without underlying disease was administered pregabalin 75mg once daily for neuropathic pain secondary to L4-L5 radiculopathy; hyponatremia developed two weeks later12). In another case, a patient with type II diabetes mellitus, nephropathy, neuropathy, and ischemic cardiomyopathy with congestive heart failure (left ventricular function, 27%) was given pregabalin for neuropathic pain. One week later, he presented with disorientation secondary to hyponatremia13). In both cases, the patients were treated with pregabalin to alleviate neuropathic pain, and the hyponatremia improved after discontinuation of pregabalin.
Pregabalin is considered among the first-line treatments for neuropathic pain, along with tricyclic antidepressants (TCA), SSRIs, and serotonin norepinephrine reuptake inhibitors (SNRI). TCAs, SSRIs, and SNRIs are well known causes of SIADH. We thought that pregabalin might enhance ADH release, or have an effect similar to the aforementioned drugs. ADH secretion results in a concentrated urine, leading to reduced urine volume. In most patients with SIADH, ingestion of water does not adequately suppress ADH, and the urine remains concentrated. This leads to water retention, which increases total body water (TBW). This increase in TBW lowers the serum sodium concentration by dilution6). In addition, the increase in TBW transiently expands the extracellular fluid (ECF) volume, and it is thought that this increased ECF volume might cause peripheral edema and weight gain. Increased ECF volume also triggers increased urinary sodium excretion, which both returns the ECF volume towards normal and further lowers the serum sodium concentration. This accounts for the euvolemic status of drug-induced SIADH.
Many medications have been associated with SIADH, and the only definitive treatment for drug-induced SIADH is the removal of the suspicious agent. Here we presented a case of SIADH that was improved by discontinuation of pregabalin. This is the first case reported in South Korea. However, we speculate that pregabalin-induced SIADH occurs more frequently than has been reported, because it appears to present with asymptomatic and mild hyponatremia. Therefore, it may be difficult to detect in the absence of laboratory studies.
In conclusion, hyponatremia should be considered and closely monitored when initiating pregabalin therapy. In addition, it is important for clinicians to suspect the drug as a potential cause of hyponatremia, particularly in the absence of another causative explanation, even though the agent is not generally known to induce SIADH.
Go to : 
References
1. Ellison DH, Berl T. The syndrome of inappropriate atidiuresis. N Engl J Med. 2007; 356(20):2064–2072. PMID: 17507705.
2. Palmer BF, Gates JR, Lader M. Causes and management of hyponatremia. Ann Pharmacother. 2003; 37:1694–1702. PMID: 14565794.

3. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967; 42:790–806. PMID: 5337379.

4. Spigset O, Hedenmalm K. Hyponatremia and the syndrome of inappropriate antidiuretic horemone secretion (SIADH) induced by psychotropic drugs. Drug Saf. 1995; 12:209–225. PMID: 7619332.
5. Foote EF. Syndrome of inappropriate antidiuretic hormone secretion and diabetes insipidus. In : Tisdale JE, Millder DA, editors. Drug-induced diseases, prevention detection and management. Bethesda, MD: American Society of Health-System Pharmacists;2005. p. 611–624.
6. Rose BD. Causes of the SIADH. In : Rose BD, editor. UpTo Date [electronic database]. Waltham, MA: 2007.
7. Page RL 2nd, Cantu M, Lindenfeld J, Hergott LJ, Lowes BD. Possible heart failure exacerbation associated with pregabalin: case discussion and literature review. J Cardiovasc Med (Hagerstown). 2008; 9(9):922–925. PMID: 18695430.

8. De Smedt RH, Jaarsma T, van den Broek SAJ, Haaijer-Ruskamp FM. Decompensation of chronic heart failure associated with pregabalin in a 73-year-old patient with post-herpetic neuralgia: a case report. Br J Clin Pharmacol. 2008; 66(2):327–328. PMID: 18507657.

9. Shim JH. Clinical Application of Alpha2-Delta Ligand. Hanyang Med Rev. 2011; 31(2):55–62.
10. Sills GJ. The mechanism of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006; 6(1):108–113. PMID: 16376147.
11. Messerli FH. Vasodilatory edema: a common side effect of antihypertensive therapy. Am J Hypertens. 2001; 14(9):978–979. PMID: 11587169.

12. Sudhagar M, Aneesh B, Nayyar I. Syndrome of inappropriate antidiuretic hormone secretion and pregabalin. J Case Rep. 2014; 4(2):408–411.
13. Blum A, Claudia S, Imad T. Hyponatremia and confusion caused by pregabalin. Isr Med Assoc J. 2009; 11:699–700. PMID: 20108561.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download