Abstract
This report describes a case of severe hypernatremia with a serum sodium concentration of 188.1mmol/L caused by exogenous salt intake. A 26-year-old man diagnosed with Crohn's disease 5 years previously visited our clinic due to generalized edema and personality changes, with aggressive behavior. He had compulsively consumed salts, ingesting approximately 154 g of salt over the last 4 days. Despite careful fluid management that included not only hypotonic fluid therapy for 8 hours but also hypertonic saline administration, his serum sodium level decreased sharply at 40.6 mmol/L; however, it returned to normal within 72-hour of treatment without any neurological deficits. Primary hypothyroidism was also diagnosed. He was discharged after 9 days from admission, with a stable serum sodium level. We have described the possibility of successful treatment in a patient with hypernatremia caused by acute salt intoxication without sustained hypotonic fluid therapy.
Go to : 
Hypernatremia caused by the disruption of water homeostasis is a commonly encountered electrolyte disorder, it frequently develops into serious complications such as hemorrhage, thrombosis and cerebral edema resulting not from the hypernatremia itself but from its inappropriate rate of sodium correction123). Because impairment of thirst or limited access to water is the main cause of sustained hypernatremia, excessive intake of salt causing severe hypernatremia is rarely diagnosed in healthy, young adults4).
Here, we have described a case of hypernatremia that developed due to exogenous intake of salt accompanied by primary hypothyroidism, which was successfully treated by intravenously infused hypotonic and hypertonic saline to allow gradual correction of serum sodium; abrupt change of sodium level was occurred nevertheless; without the occurrence of any neurological sequelae.
Go to : 
A 26-year-old man diagnosed with Crohn's disease (CD) 5 years previously was admitted to our clinic for generalized edema, sudden weight gain, 10 kg over 2 weeks, reaching to 59 kg and personality changes with aggressive behavior. Reportedly, he had muttered something to himself and then suffered loss of bowel control 4 hour before presentation. The patient was abnormally obsessed with ingestion of salt voluntarily for the past 4 days, based on mistaken information that salt consumption could relieve generalized edema. The total consumption was estimated to be approximately 154 g of sun-dried salt.
On arrival to our emergency department, the patient was afebrile with a blood pressure of 90/60 mmHg, respiratory rate of 16, heart rate of 87, and the neurological examination was unremarkable. Initial laboratory tests yielded the following results: serum sodium, 188.1 mmol/L (normal, 138–148 mmol/L); potassium, 3.32 mmol/L(normal, 3.5–5.3 mmol/L); chloride, 160.9 mmol/L(normal, 100–110 mmol/L); bicarbonate, 23.7 mmol/L (normal, 20–28 mmol/L); anion gap, 3.5; calculated serum osmolality, 380 mOsm/kg (normal, 275–300 mOsm/kg); urine osmolality, 817 mosm/kg (normal, 300–800 mOsm/kg); osmolar gap, −5 mOsm/kg; total protein, 3.4 g/dL(normal, 6.0–8.0 g/dL); and albumin 1.5 g/dL (normal, 3.3–5.2 g/dL).
Hypernatremia caused by salt intoxication was diagnosed and we set up the maximal rate of correction of sodium concentration of 10 mmol/L/day due to the ambiguous duration of the hypernatremia, using 5% dextrose as the hypotonic fluid administered at the rate of 150 mL/hour. The urine output of the patient was maintained at approximately 30 mL/hour.
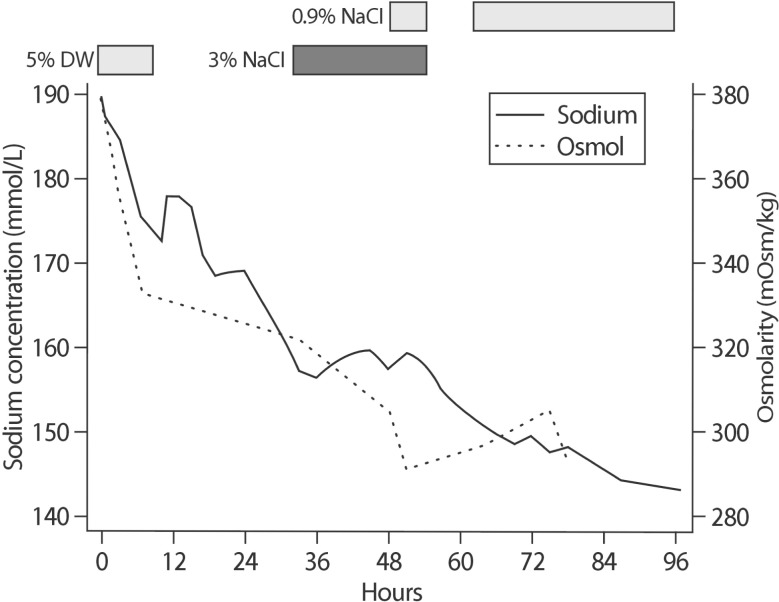
Within 3 hours, a decrease of approximately 4 mmol/L in the serum sodium level was noted (184.8 mmol/L) and the infusion rate was adjusted to 40 mL/hour (Fig. 1). At 5-hour later, the serum sodium level had decreased sharply to 175.1 mmol/L and we stopped the administration of the hypotonic fluid. Although we discontinued the fluid therapy, the serum sodium level decreased by 30 mmol/L, within 24 hours, reaching 157.4 mmol/L. Therefore, we restarted an infusion of 3% saline and the serum sodium level stabilized around 157.5 to 159.4 mmol/L for 16 hours. Then, the infusion rate of 3% saline was reduced from 100 mL/hour to 50 mL/hour with concomitant addition of 0.9% saline at 50 mL/hour (Fig. 1). Approximately 6 hours later, we stopped the administration of 3% and 0.9% saline and administered 20 mg of furosemide to control the generalized edema. At this point, the patient had gained 5 kg in weight, weighing 64 kg.
At 6 hours after cessation of saline infusion, the serum sodium level decreased from 154.9 to 151.4 mmol/L. However, the patient became hypotensive (70/40 mmHg) and required an infusion of 0.9% saline (100 mL/hour). Despite restarting the 0.9% saline infusion, the serum sodium level decreased continually and reached 149.7 mmol/L at 3 hours later. After 72-hour of hospitalization, his sodium concentration had returned to normal level (147.5 mmol/L) through treatment with hypertonic and isotonic saline infusion (Fig. 1). To exclude other causes of generalized edema, thyroid function test was performed, revealing primary hypothyroidism(Free T4 0.691 ng/dL, TSH 10.50 µIU/mL, and T3 73.76 ng/dL). He was discharged after 9 days from admission, with a stable serum sodium level of 143.0 mmol/L. No neurological abnormalities were found throughout the treatment course. The patient was prescribed 50 µg of levothyroxine daily and his sodium level stays within the normal range after discharge (139.5-142.3 mmol/L).
Go to : 
Inappropriate correction of hypernatremia with a rapid fall in serum sodium levels is associated with a high fatality rate5). The rate adopted for lowering the serum sodium concentration depends on the rapidity of development of hypernatremia. A differentiation between acute and chronic hypernatremia can be made if the rise in serum sodium has a documented onset within the last 48 hours6). In general, individuals with hypernatremia should be managed such that the reduction rate of serum sodium is approximately 0.5 mmol/L/day, not exceeding 10 mmol/L/day78). However, in patients with hypernatremia that has developed over a period of hours, rapid correction (1 mmol/L/hour) improves the prognosis without increasing the risk of cerebral edema because accumulated electrolytes are rapidly extruded from brain cells910). In patients with hypernatremia of longer or unknown duration, a slower pace of correction is prudent, because the full dissipation of accumulated brain solutes occurs over a period of several days1011). However, in the present case, the abrupt reduction in the serum sodium concentration did not cause any neurological problems. Although the duration of the salt ingestion was over 48 hours, we assumed that the hypernatremia in this case was a relatively acute event considering the abrupt behavioral changes that are considered as symptoms of hypernatremia, therefore a sudden correction of the sodium level in acute hypernatremia would not lead to fatal problems. Our case report raises the question whether the established classification of hypernatremia, distinguishing between acute and chronic hypernatremia based on the “48 hour”, should be an absolute standard when deciding the correction rate and its adjustment. Indeed, no prospective studies have validated the recommendations for the correction rates in acute and chronic hypernatremia12).
Another potential explanation was that the primary hypothyroidism, which led to the impairment of water secretion as well as an increase of urinary sodium excretion, disturbed the expected rate of correction. The thyroid hormone affects renal hemodynamics, the renin-angiotensin-aldosterone system and renal electrolyte handling13). There is a proven association between hypothyroidism and hyponatremia as a consequence of inability to excrete free water load, caused by both a decrease in the delivery of water to the distal nephron14) and enhanced renal water retention mediated by excess vasopressin secretion. However, little is known regarding the effect of hypothyroidism on the rate of sodium correction in the treatment of hypernatremic patients. Additionally, considering CD-associated mucosal inflammation may cause hyponatremia due to reversal of sodium-water flux in colonic epithelium15), CD may not lead to aggravation of hypernatremia in our case.
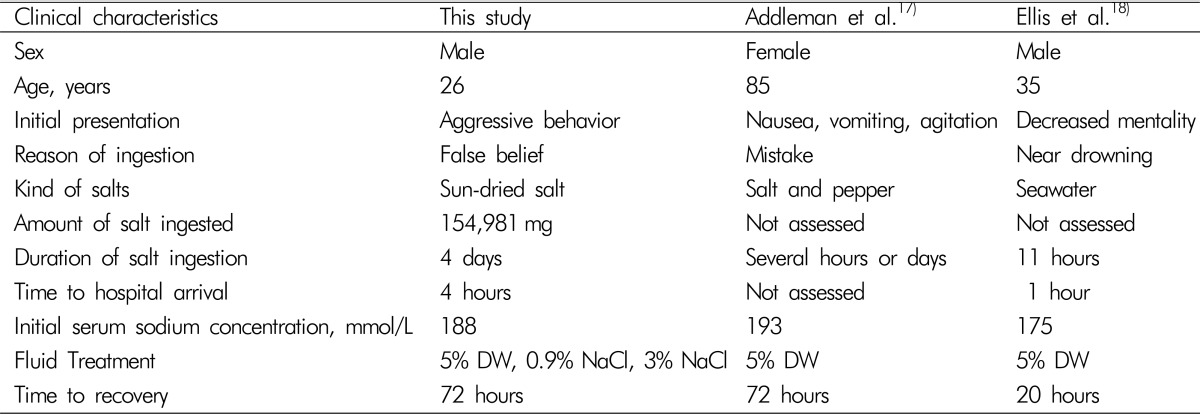
It has been known that the estimated fatal amount is 1 g sodium chloride per kg body weight16), leading to a rise in blood sodium concentration of about 30 mmol/L. In literature, 19 cases of severe hypernatremia due to salt ingestion in adults were identified. All but two patients have died. All dead patients represented a short period of salt ingestion, even a few minutes. Otherwise, the surviving patients had a relatively longer duration of sodium exposure than non-surviving cases, with considerably different sodium concentrations, though1718). In surviving cases, Addleman et al.17) reported a longer period of salt intake than those reported by Ellis et al.18), several days and 11 hours, respectively (Table 1). Addleman et al. showed an abrupt decrease in the serum sodium level, from an initial value of 193 mmol/L to the normal range within 72 hours, with no neurological sequelae similar to the course of recovery in our case. However they did not prescribe hypertonic saline (Table 1). Our case can be differentiated from previous cases of hypernatremia over a longer duration and successful survival despite aggressive hypotonic fluid replacement4).
To our knowledge, this is the first case a of patient with CD and newly diagnosed primary hypothyroidism with severe symptomatic hypernatremia due to exogenous salt intoxication, successfully treated without sustained hypotonic fluid therapy along with infusion of hypertonic saline. We suggest that the existing standards for hypernatremia, classified as acute and chronic based on 48 hour onset6), require revision. A novel approach for the setting the target rate of sodium correction could be helpful in treating hypernatremia.
Go to : 
Acknowledgments
The authors declare no relevant financial interests. This article has not been published previously and will not be submitted for publication elsewhere.
Go to : 
Notes
Conflict of Interest Disclosure: This manuscript has not published previously, and will not be submitted for publication elsewhere.
Go to : 
References
1. Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med. 1987; 107:309–319. PMID: 3619220.
2. Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996; 124:197–203. PMID: 8533994.

4. Ofran Y, Lavi D, Opher D, Weiss TA, Elinav E. Fatal voluntary salt intake resulting in the highest ever documented sodium plasma level in adults (255 mmol L-1): a disorder linked to female gender and psychiatric disorders. J Intern Med. 2004; 256:525–528. PMID: 15554954.

5. Chisti MJ, Pietroni MA, Smith JH, Bardhan PK, Salam MA. Predictors of death in under-five children with diarrhoea admitted to a critical care ward in an urban hospital in Bangladesh. Acta Paediatr. 2011; 100:e275–e279. PMID: 21627690.

6. Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013; 28:216.e11–216.e20.

7. Blum D, Brasseur D, Kahn A, Brachet E. Safe oral rehydration of hypertonic dehydration. J Pediatr Gastroenterol Nutr. 1986; 5:232–235. PMID: 3958850.

8. Kahn A, Brachet E, Blum D. Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med. 1979; 5:27–31. PMID: 35558.

9. Palevsky PM. Hypernatremia. Semin Nephrol. 1998; 18:20–30. PMID: 9459286.
10. Lien YH, Shapiro JI, Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990; 85:1427–1435. PMID: 2332498.

11. Hogan GR, Dodge PR, Gill SR, Master S, Sotos JF. Pathogenesis of seizures occurring during restoration of plasma tonicity to normal in animals previously chronically hypernatremic. Pediatrics. 1969; 43:54–64. PMID: 5764069.

12. Nur S, Khan Y, Boroujerdi H. Hypernatremia: correction rate and hemodialysis. Case Rep Med. 2014; 2014:736073. PMID: 25431600.

13. Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012; 23:22–26. PMID: 22021708.

14. Derubertis FR Jr, Michelis MF, Bloom ME, Mintz DH, Field JB, Davis BB. Impaired water excretion in myxedema. Am J Med. 1971; 51:41–53. PMID: 5570319.

15. Barkas F, Liberopoulos E, Kei A, Elisaf M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol. 2013; 26:23–28. PMID: 24714322.
16. Turk EE, Schulz F, Koops E, Gehl A, Tsokos M. Fatal hypernatremia after using salt as an emetic--report of three autopsy cases. Leg Med (Tokyo). 2005; 7:47–50. PMID: 15556015.
17. Addleman M, Pollard A, Grossman RF. Survival after severe hypernatremia due to salt ingestion by an adult. Am J Med. 1985; 78:176–178. PMID: 3966483.

18. Ellis RJ. Severe hypernatremia from sea water ingestion during near-drowning in a hurricane. West J Med. 1997; 167:430–433. PMID: 9426487.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download