Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease. Hypertension is common and occurs before decline in renal function. However, the coexistence of hypertension and hypokalemia is rare in ADPKD patients. We report on a 32-year-old woman with secondary aldosteronism. Magnetic resonance imaging of the renal arteries revealed multiple cysts of varying sizes in both the kidneys and the liver, compatible with ADPKD. Increased reninangiotensin-aldosterone system activity was secondary to cyst expansion. After initiation of angiotensin II receptor blocker, her blood pressure was controlled without additional requirement of potassium.
Secondary hypertension is defined as hypertension that results from an identifiable etiology. Only 5-10% of hypertension cases are thought to have a secondary cause, whereas the majority of hypertensive patients have essential (idiopathic or primary) hypertension1). Because hypertension can be controlled by specific medication or surgery, it is thus important to identify certain clinical suspicions that could suggest the secondary form of hypertension.
One of the clinical features indicative of secondary hypertension is hypokalemia due to mineralocorticoid excess. Hypokalemia may be combined with metabolic alkalosis and excessive urinary sodium excretion. Disorders with mineralocorticoid excess can be divided into three categories. First, in the high aldosterone-low renin category, the diagnosis is primary aldosteronism. High aldosteronehigh renin, known as secondary aldosteronism, includes malignant hypertension, renovascular hypertension, and renin-producing tumor. In the low aldosterone-low renin category, the diagnoses to consider are apparent mineralocorticoid excess, congenital adrenal hyperplasia, Cushing's syndrome, deoxycorticosterone-producing tumor, exogenous mineralocorticoid intake, and Liddle's syndrome.
Autosomal dominant polycystic kidney disease (ADPKD) is a common hereditary renal disease affecting approximately 12.5 million people worldwide. It is characterized by the gradual growth of multiple renal cysts, hypertension and, eventually, end-stage renal disease (ESRD), accounting for 5-10% of ESRD cases23). Hypertension is the early and frequent manifestation of ADPKD. It appears in 50-70% of cases before a noticeable reduction in glomerular filtration, with the average age of onset of 30 years4). ADPKD in patients with hypertension leads to a great and rapid loss of renal function5). Up-regulation of the reninangiotensin-aldosterone system(RAAS) plays a major role in the pathogenesis of hypertension in ADPKD. However, the coexistence of hypertension and hypokalemia is very rare in ADPKD patients. We illustrate a case with hypertension and hypokalemia who was finally diagnosed with ADPKD and successfully corrected hypokalemia and hypertension with angiotensin II receptor blocker (ARB).
A 32-year-old Asian female was referred to an endocrinologist for evaluation of hypertension and hypokalemia. Blood pressure of 170/120mmHg was found during a routine annual examination. She was asymptomatic and did not take diuretic, liquorice or herbs. She did not have diarrhea, vomiting, history of chronic alcohol intake or toxin exposure. Her parents had hypertension starting in late adulthood. She had no family history of stroke, renal disease, periodic paralysis or hypokalemia. Her electrolytes were notable for serum potassium of 3 mmol/L (mEq/L) with spot urine potassium concentration of 23mmol/L. Despite taking a combination of antihypertensive drugs of amlodipine 10mg daily and atenolol 50mg daily, her blood pressure remained 130/100-150/110mmHg.
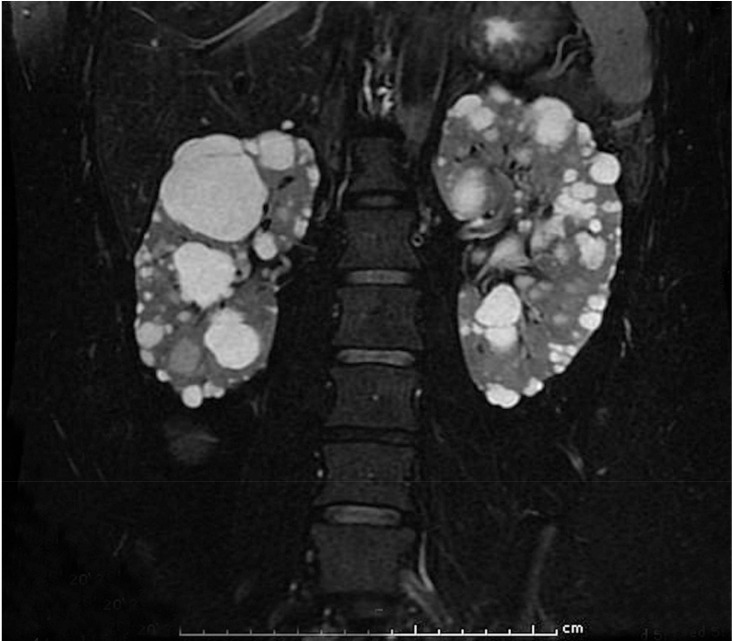
On examination, the patient's blood pressure was 134/96 mmHg and heart rate was 104 beats/min. Clinical examination findings were unremarkable except for being overweight according to Asian criteria (body mass index, 23.4 kg/m2). No Cushingoid appearance was detected. Auscultation showed no renal bruit. Laboratory evaluation showed hypokalemia and normal renal function [sodium 140mmol/L (136-145), potassium 3.31mmol/L (3.5-5.1), chloride 106mmol/L (98-107), carbon dioxide 26.4mmol/L (22-29), blood urea nitrogen 19mg/dL (7-18), creatinine 0.77mg/dL (0.51-0.95)]. Urinalysis showed 3-5 red blood cells per high-power field, without protein and glucose. She required up to 1.5 g of oral potassium chloride daily to maintain normokalemia. Supine plasma renin activity and aldosterone concentrations were 1.41 ng/mL/h (reference range, supine position 0.23-3.33 and seated position 0.36-3.84 ng/mL/h) and 25.2 ng/dL (reference range, supine position 1-16 and seated position 2.5-31.5 ng/dL), with an aldosterone/renin ratio of 17.87 (primary hyperaldosteronism, ratio >20; secondary hyper- aldosteronism, ratio ~10). Clinical and laboratory data favored a diagnosis of secondary aldosteronism. Magnetic resonance imaging of renal arteries was initially planned to exclude renal artery stenosis, but the examination revealed multiple cysts of varying sizes in both the kidneys and the liver, consistent with ADPKD(Fig. 1). There was no adrenal mass or renal artery stenosis.
After the diagnosis of ADPKD had been established, the patient received valsartan 80mg daily without further requirement of potassium(Table 1). After a 4-year follow-up, her blood pressure was controlled within the normal range, and she had no further episodes of hypokalemia.
We demonstrate a patient with ADPKD discovered by work-up of secondary aldosteronism. In addition, we show the benefit of ARB for treatment of hypertension and hypokalemia.
Increased blood pressure in patients with ADPKD have been attributed to several causes, including activation of the RAAS, increase in sympathetic nervous system activity, endothelial dysfunction, increased endothelin-1, and arterial stiffness. The most important factor is the activation of the RAAS, possibly caused by renal vascular compression by cyst expansion, leading to bilateral renal ischemia. Plasma renin activity and plasma aldosterone concentration in hypertensive patients with ADPKD were significantly higher than in matched patients with essential hyperten- sion6). In addition, immunohistochemical studies and clinical studies support a major role of the intrarenal RAAS in the pathogenesis of hypertension in patients with ADPKD. Activation of the RAAS may enhance renal cyst formation and enlargement by means of its mitogenic effects7).
However, hypokalemia is a rare manifestation in ADPKD patients. Renal impairment due to ADPKD could mask hypokalemia. Additionally, activation and dysregulation of the intrarenal RAAS is sufficient to result in increased blood pressure without affecting the circulating RAAS8). In our patient, normal plasma renin activity and a low aldosterone/renin ratio favored a diagnosis of secondary aldosteronism. The coexistence of hypertension and hypokalemia in secondary aldosteronism is an uncommon presentation in ADPKD patients; only two cases have been reported910). There are other case reports of ADPKD complicated by the coincidence of primary aldosteronism1112131415). The diagnosis included hyperaldosteronemia and hyporeninemia. Even though the identification of adrenal adenoma may be obscured by adjacent renal cysts, adrenal venous sampling can confirm the site of aldosterone-producing adenoma. Excessive aldosterone aggravates cyst formation and/or progression1617). Among patients with primary aldosteronism, either adrenalectomy or spironolactone resulted in the abrogation of newly developed renal cysts on follow-up ultrasonographic imaging17).
Considering that RAAS plays an important role in the pathophysiology of hypertension in ADPKD, treatment with angiotensin-converting enzyme inhibitor (ACEI) or ARB has been suggested to be the first-line of treatment. Increased renal plasma flow and decreased renal vascular resistance were found in hypertensive patients with ADPKD after administration of enalapril6). This rationale was supported by recent data from large, randomized, double-blind, placebo-controlled Halt Progression of Polycystic Kidney Disease (HALT-PKD) trials1819). Theoretically, dual therapy of ACEI and ARB should improve renal outcomes by circumventing the compensatory feedback response that generates more angiotensin II when a single blocker is used. Data from HALT-PKD trials showed that combination therapy was not more effective than monotherapy with an RAAS blocker1819). The role of the direct renin inhibitor, aliskiren, is not yet established in ADPKD patients; however, it could be relevant given that this is the only RAAS inhibitor which also reduces plasma renin910). To date, there is no head-to-head comparison study between ACEI and ARB for the renal or cardiovascular outcomes in ADPKD patients. There is no available evidence on the potential benefits of adding an aldosterone antagonist to ACEIs or ARBs.
Previous reports of ADPKD cases with secondary aldosteronism were managed with a direct renin inhibitor (aliskiren)910), while our case was managed with ARB. Hypertension was successfully controlled without a potassium supplement in all cases. ARB blocks the binding of angiotensin II to the angiotensin-1 receptor, which leads to increments in plasma renin activity and levels of angiotensin I and angiotensin II. However, this elevation of precursors does not overcome the receptor blockade, as evidenced by a persistent decrease in both blood pressure and plasma aldosterone concentrations20). Either ACEI or ARB, not only direct renin inhibitor, could be the drug of choice for managing ADPKD with secondary aldosteronism. However, further study is required to compare the effectiveness of hypertension treatment among RAAS- blocking agents in ADPKD with secondary aldosteronism.
In summary, hypertension with hypokalemia is an uncommon presentation that clinicians should be aware of in ADPKD. RAAS activation plays an important role in the pathogenesis of hypertension in ADPKD patients. Understanding the exact mechanism of hypertension will contribute to effective blood pressure control, as observed in this case.
References
1. Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014; 35:1245–1254. PMID: 24366917.

2. Reule S, Sexton DJ, Solid CA, Chen SC, Collins AJ, Foley RN. ESRD from autosomal dominant polycystic kidney disease in the United States, 2001-2010. Am J Kidney Dis. 2014; 64:592–599. PMID: 25134777.

3. Spithoven EM, Kramer A, Meijer E, et al. : Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival--an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014; 29(Suppl 4):15–25. PMID: 23986077.
4. Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol. 2001; 12:194–200. PMID: 11134267.

5. Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992; 41:1311–1319. PMID: 1614046.

6. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990; 323:1091–1096. PMID: 2215576.

7. Thomas W, Dooley R, Harvey BJ. Aldosterone as a renal growth factor. Steroids. 2010; 75:550–554. PMID: 19782095.

8. Doulton TW, Saggar-Malik AK, et al. The effect of sodium and angiotensin-converting enzyme inhibition on the classic circulating renin-angiotensin system in autosomal-dominant polycystic kidney disease patients. J Hypertens. 2006; 24:939–945. PMID: 16612257.

9. Amico P, Kalbermatter S, Kiss D. Aliskiren corrects recurrent hyperreninemia and hyperaldosteronism in autosomal dominant polycystic kidney disease. Clin Nephrol. 2009; 72:237–239. PMID: 19761733.
10. Chow KM, Ma RC, Szeto CC, Li PK. Polycystic kidney disease presenting with hypertension and hypokalemia. Am J Kidney Dis. 2012; 59:270–272. PMID: 21962616.

11. Bobrie G, Sirieix ME, Day M, Landais P, Lacombe M, Grunfeld JP. Autosomal dominant polycystic kidney disease with primary hyperaldosteronism. Nephrol Dial Transplant. 1992; 7:647–650. PMID: 1323077.

12. Gejyo F, Ishida K, Arakawa M. Autosomal dominant polycystic kidney disease complicated by primary aldosteronism. Case report and review of the literature. Am J Nephrol. 1994; 14:236–238. PMID: 7977490.
13. Liou HH, Tsai SC, Chen WJ, Huang TP, Huang WJ, Chen KK. The association of aldosterone-producing adrenal adenoma in a patient with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1994; 23:739–742. PMID: 8172219.

14. Kao CC, Wu VC, Kuo CC, et al. Delayed diagnosis of primary aldosteronism in patients with autosomal dominant polycystic kidney diseases. J Renin Angiotensin Aldosterone Syst. 2013; 14:167–173. PMID: 22791703.

15. Peixoto AJ. A young patient with a family history of hypertension. Clin J Am Soc Nephrol. 2014; 9:2164–2172. PMID: 25092599.

16. Ogasawara M, Nomura K, Toraya S, et al. Clinical implications of renal cyst in primary aldosteronism. Endocrine J. 1996; 43:261–268. PMID: 8886619.

17. Novello M, Catena C, Nadalini E, et al. Renal cysts and hypokalemia in primary aldosteronism: results of long-term follow-up after treatment. J Hypertens. 2007; 25:1443–1450. PMID: 17563567.

18. Schrier RW, Abebe KZ, Perrone RD, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014; 371:2255–2266. PMID: 25399733.

19. Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014; 371:2267–2276. PMID: 25399731.

20. Grossman E, Peleg E, Carroll J, Shamiss A, Rosenthal T. Hemodynamic and humoral effects of the angiotensin II antagonist losartan in essential hypertension. Am J Hypertens. 1994; 7:1041–1044. PMID: 7702796.

Fig. 1
Magnetic resonance imaging of the renal artery revealed innumerable cysts, causing diffuse enlargement of both kidneys. The cysts ranged from subcentimeter size to the largest diameter of 5.3 cm. The largest cyst is seen at the upper pole of the right kidney, which had thin septation. Also, there were multiple small hepatic cysts ranging from tiny to 1.1 cm in diameter (not shown). Renal arteries and adrenal glands were normal (not shown).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download